Huntingtin coordinates the dynein-mediated dynamic positioning of endosomes and lysosomes
- PMID: 21169558
- PMCID: PMC3038646
- DOI: 10.1091/mbc.E10-03-0233
Huntingtin coordinates the dynein-mediated dynamic positioning of endosomes and lysosomes
Abstract
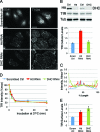
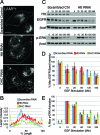
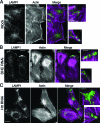
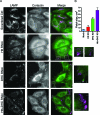
Huntingtin (Htt) is a membrane-associated scaffolding protein that interacts with microtubule motors as well as actin-associated adaptor molecules. We examined a role for Htt in the dynein-mediated intracellular trafficking of endosomes and lysosomes. In HeLa cells depleted of either Htt or dynein, early, recycling, and late endosomes (LE)/lysosomes all become dispersed. Despite altered organelle localization, kinetic assays indicate only minor defects in intracellular trafficking. Expression of full-length Htt is required to restore organelle localization in Htt-depleted cells, supporting a role for Htt as a scaffold that promotes functional interactions along its length. In dynein-depleted cells, LE/lysosomes accumulate in tight patches near the cortex, apparently enmeshed by cortactin-positive actin filaments; Latrunculin B-treatment disperses these patches. Peripheral LE/lysosomes in dynein-depleted cells no longer colocalize with microtubules. Htt may be required for this off-loading, as the loss of microtubule association is not seen in Htt-depleted cells or in cells depleted of both dynein and Htt. Inhibition of kinesin-1 relocalizes peripheral LE/lysosomes induced by Htt depletion but not by dynein depletion, consistent with their detachment from microtubules upon dynein knockdown. Together, these data support a model of Htt as a facilitator of dynein-mediated trafficking that may regulate the cytoskeletal association of dynamic organelles.
Figures




References
-
- Aridor M, Hannan LA. Traffic jam: a compendium of human diseases that affect intracellular transport processes. Traffic. 2000;1:836–851. - PubMed
-
- Aridor M, Hannan LA. Traffic jams II: an update of diseases of intracellular transport. Traffic. 2002;3:781–790. - PubMed
-
- Atwal RS, Xia J, Pinchev D, Taylor J, Epand RM, Truant R. Huntingtin has a membrane association signal that can modulate huntingtin aggregation, nuclear entry and toxicity. Hum Mol Genet. 2007;16:2600–2615. - PubMed
-
- Brown CL, Maier KC, Stauber T, Ginkel LM, Wordeman L, Vernos I, Schroer TA. Kinesin-2 is a motor for late endosomes and lysosomes. Traffic. 2005;6:1114–1124. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

