Selective reduction in neural responses to high calorie foods following gastric bypass surgery
- PMID: 21169809
- PMCID: PMC3128512
- DOI: 10.1097/SLA.0b013e318203a289
Selective reduction in neural responses to high calorie foods following gastric bypass surgery
Abstract
Objective: To investigate changes in neural activation and desire to eat in response to appetitive cues from pre- to postbariatric surgery for obesity.
Background: Roux-en-Y gastric bypass (RYGB) is the most common bariatric procedure. However, the mechanisms of action in RYGB are not well understood. A significant proportion of the resulting reduction in caloric intake is unaccounted for by the restrictive and malabsorptive mechanisms and is thought to be mediated by neuroendocrine function. Numerous investigations of postsurgical changes in gut peptides have resulted; however, changes in neural activation after RYGB surgery have not been previously investigated.
Methods: Functional magnetic resonance imaging and verbal rating scales were used to assess brain activation and desire to eat in response to high- and low-calorie food cues in 10 female patients 1-month pre- and post-RYGB surgery.
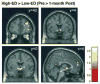
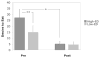
Results: Postsurgical reductions in brain activation were found in key areas within the mesolimbic reward pathway, which were significantly more pronounced in response to food cues that were high (vs. low) in caloric density. These changes mirrored concurrent postsurgical reductions in desire to eat, which were also greater in response to food cues that were high versus low in caloric density (P = 0.007).
Conclusions: Findings support the contention that RYGB surgery leads to substantial changes in neural responses to food cues encountered in the environment, provide a potential mechanism for the selective reduction in preferences for high-calorie foods, and suggest partial neural mediation of changes in caloric intake seen after RYGB surgery.
Figures

References
-
- Ogden CL, Carroll MD, Curtin LR, et al. Prevalence of overweight and obesity in the United States, 1999–2004. JAMA. 2006;295(13):1549–1555. - PubMed
-
- Buchwald H, Avidor Y, Braunwald E, et al. Bariatric surgery: a systematic review and meta-analysis. JAMA. 2004;292:1724–1737. - PubMed
-
- Poves I, Cabrera M, Maristany C, et al. Gastrointestinal quality of life after laparoscopic Roux-en-Y gastric bypass. Obes Surg. 2006;16:19–23. - PubMed
-
- Vincent RP, le Roux CW. Changes in gut hormones after bariatric surgery. Clin Endocrinol. 2008;69(2):173–179. - PubMed
-
- Tadross JA, le Roux CW. The mechanisms of weight loss after bariatric surgery. Int J Obes. 2009;33:S28–S32. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

