NF-κB addiction and its role in cancer: 'one size does not fit all'
- PMID: 21170083
- PMCID: PMC3141287
- DOI: 10.1038/onc.2010.566
NF-κB addiction and its role in cancer: 'one size does not fit all'
Abstract
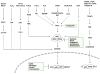
Activation of nuclear factor (NF)-κB, one of the most investigated transcription factors, has been found to control multiple cellular processes in cancer including inflammation, transformation, proliferation, angiogenesis, invasion, metastasis, chemoresistance and radioresistance. NF-κB is constitutively active in most tumor cells, and its suppression inhibits the growth of tumor cells, leading to the concept of 'NF-κB addiction' in cancer cells. Why NF-κB is constitutively and persistently active in cancer cells is not fully understood, but multiple mechanisms have been delineated including agents that activate NF-κB (such as viruses, viral proteins, bacteria and cytokines), signaling intermediates (such as mutant receptors, overexpression of kinases, mutant oncoproteins, degradation of IκBα, histone deacetylase, overexpression of transglutaminase and iNOS) and cross talk between NF-κB and other transcription factors (such as STAT3, HIF-1α, AP1, SP, p53, PPARγ, β-catenin, AR, GR and ER). As NF-κB is 'pre-active' in cancer cells through unrelated mechanisms, classic inhibitors of NF-κB (for example, bortezomib) are unlikely to mediate their anticancer effects through suppression of NF-κB. This review discusses multiple mechanisms of NF-κB activation and their regulation by multitargeted agents in contrast to monotargeted agents, thus 'one size does not fit all' cancers.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. - PubMed
-
- Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. - PubMed
-
- Aggarwal S, Ichikawa H, Takada Y, Sandur SK, Shishodia S, Aggarwal BB. Curcumin (diferuloylmethane) down-regulates expression of cell proliferation and antiapoptotic and metastatic gene products through suppression of IkappaBalpha kinase and Akt activation. Mol Pharmacol. 2006;69:195–206. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

