Sex in an Evolutionary Perspective: Just Another Reaction Norm
- PMID: 21170116
- PMCID: PMC2987205
- DOI: 10.1007/s11692-010-9101-8
Sex in an Evolutionary Perspective: Just Another Reaction Norm
Abstract
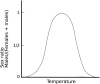
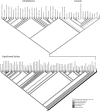
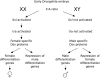
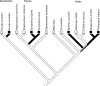
It is common to refer to all sorts of clear-cut differences between the sexes as something that is biologically almost inevitable. Although this does not reflect the status of evolutionary theory on sex determination and sexual dimorphism, it is probably a common view among evolutionary biologists as well, because of the impact of sexual selection theory. To get away from thinking about biological sex and traits associated with a particular sex as something static, it should be recognized that in an evolutionary perspective sex can be viewed as a reaction norm, with sex attributes being phenotypically plastic. Sex determination itself is fundamentally plastic, even when it is termed "genetic". The phenotypic expression of traits that are statistically associated with a particular sex always has a plastic component. This plasticity allows for much more variation in the expression of traits according to sex and more overlap between the sexes than is typically acknowledged. Here we review the variation and frequency of evolutionary changes in sex, sex determination and sex roles and conclude that sex in an evolutionary time-frame is extremely variable. We draw on recent findings in sex determination mechanisms, empirical findings of morphology and behaviour as well as genetic and developmental models to explore the concept of sex as a reaction norm. From this point of view, sexual differences are not expected to generally fall into neat, discrete, pre-determined classes. It is important to acknowledge this variability in order to increase objectivity in evolutionary research.
Figures
References
-
- Andersson M. Sexual selection. Princeton: Princeton University Press; 1994.
-
- Bagemihl B. Biological exuberance: Animal homosexuality and natural diversity. London: Profile Books; 1999.
-
- Bull JJ. Sex determination in reptiles. The Quarterly Review of Biology. 1980;55:4–21. doi: 10.1086/411613. - DOI
