A scalable approach for discovering conserved active subnetworks across species
- PMID: 21170309
- PMCID: PMC3000367
- DOI: 10.1371/journal.pcbi.1001028
A scalable approach for discovering conserved active subnetworks across species
Abstract
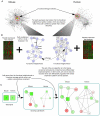
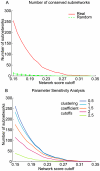
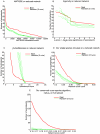
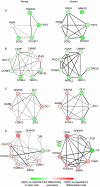
Overlaying differential changes in gene expression on protein interaction networks has proven to be a useful approach to interpreting the cell's dynamic response to a changing environment. Despite successes in finding active subnetworks in the context of a single species, the idea of overlaying lists of differentially expressed genes on networks has not yet been extended to support the analysis of multiple species' interaction networks. To address this problem, we designed a scalable, cross-species network search algorithm, neXus (Network-cross(X)-species-Search), that discovers conserved, active subnetworks based on parallel differential expression studies in multiple species. Our approach leverages functional linkage networks, which provide more comprehensive coverage of functional relationships than physical interaction networks by combining heterogeneous types of genomic data. We applied our cross-species approach to identify conserved modules that are differentially active in stem cells relative to differentiated cells based on parallel gene expression studies and functional linkage networks from mouse and human. We find hundreds of conserved active subnetworks enriched for stem cell-associated functions such as cell cycle, DNA repair, and chromatin modification processes. Using a variation of this approach, we also find a number of species-specific networks, which likely reflect mechanisms of stem cell function that have diverged between mouse and human. We assess the statistical significance of the subnetworks by comparing them with subnetworks discovered on random permutations of the differential expression data. We also describe several case examples that illustrate the utility of comparative analysis of active subnetworks.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

References
-
- van 't Veer LJ, Dai H, van de Vijver MJ, He YD, Hart AA, et al. Gene expression profiling predicts clinical outcome of breast cancer. Nature. 2002;415:530–536. - PubMed
-
- Sladek R, Rocheleau G, Rung J, Dina C, Shen L, et al. A genome-wide association study identifies novel risk loci for type 2 diabetes. Nature. 2007;445:881–885. - PubMed
-
- Ideker T, Ozier O, Schwikowski B, Siegel AF. Discovering regulatory and signalling circuits in molecular interaction networks. Bioinformatics. 2002;18:S233–240. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

