Epidemiology of rotavirus infection among young children with acute diarrhoea in Burkina Faso
- PMID: 21171984
- PMCID: PMC3013080
- DOI: 10.1186/1471-2431-10-94
Epidemiology of rotavirus infection among young children with acute diarrhoea in Burkina Faso
Abstract
Background: In anticipation of vaccine introduction, we assessed epidemiology of rotavirus disease among children visiting medical centre due to acute diarrhoea in Ouagadougou, Burkina Faso.
Methods: Between November 2008 and February 2010, stool specimens from 447 children less than 5 years of age suffering from diarrhoea were tested for the presence of rotavirus by antigen detection using an immunochromatographic test. Sociodemographic, environmental and clinical factors were assessed during the study.
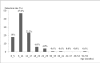
Results: Rotavirus antigen was detected in 151 (33.8%) of the patients. Most of the cases (94.2%) were in children < 24 months of age. Fever and vomiting were the symptoms most commonly reported in association with rotavirus diarrhoea and the patients were often hospitalized. Rotavirus-associated diarrhoea occurred mostly during the season from December to April (dry season). Rotavirus infection was significantly less frequent in breast-fed than among bottle-fed babies.
Conclusions: The results of this study underscore the need to control rotavirus infections among young children in Burkina Faso and may argue a decision on the introduction of rotavirus vaccine in Burkina Faso.
Figures

References
-
- Phua KB, Emmanuel SC, Goh P, Quak SH, Lee BW, Han HH, Ward RL, Bernstein DI, De Vos B, Bock HL. A rotavirus vaccine for infants: the Asian experience. Ann Acad Med Singapore. 2006;35(1):38–47. - PubMed
-
- Ruiz-Palacios GM, Pérez-Schael I, Velázquez FR, Abate H, Breuer T, Clemens SC, Cheuvart B, Espinoza F, Gillard P, Innis BL, Cervantes Y, Linhares AC, López P, Macías-Parra M, Ortega-Barría E, Richardson V, Rivera-Medina DM, Rivera L, Salinas B, Pavía-Ruz N, Salmerón J, Rüttimann R, Tinoco JC, Rubio P, Nuñez E, Guerrero ML, Yarzábal JP, Damaso S, Tornieporth N, Sáez-Llorens X, Vergara RF, Vesikari T, Bouckenooghe A, Clemens R, De Vos B, O'Ryan M. Human Rotavirus Vaccine Study Group. Safety and efficacy of an attenuated vaccine against severe rotavirus gastroenteritis. N Engl J Med. 2006;354:11–22. doi: 10.1056/NEJMoa052434. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

