Abnormal nociception and opiate sensitivity of STOP null mice exhibiting elevated levels of the endogenous alkaloid morphine
- PMID: 21172011
- PMCID: PMC3017033
- DOI: 10.1186/1744-8069-6-96
Abnormal nociception and opiate sensitivity of STOP null mice exhibiting elevated levels of the endogenous alkaloid morphine
Abstract
Background: Mice deficient for the stable tubule only peptide (STOP) display altered dopaminergic neurotransmission associated with severe behavioural defects including disorganized locomotor activity. Endogenous morphine, which is present in nervous tissues and synthesized from dopamine, may contribute to these behavioral alterations since it is thought to play a role in normal and pathological neurotransmission.
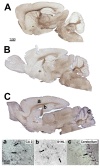
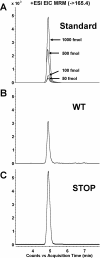
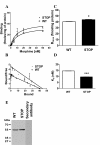
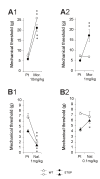
Results: In this study, we showed that STOP null brain structures, including cortex, hippocampus, cerebellum and spinal cord, contain high endogenous morphine amounts. The presence of elevated levels of morphine was associated with the presence of a higher density of mu opioid receptor with a higher affinity for morphine in STOP null brains. Interestingly, STOP null mice exhibited significantly lower nociceptive thresholds to thermal and mechanical stimulations. They also had abnormal behavioural responses to the administration of exogenous morphine and naloxone. Low dose of morphine (1 mg/kg, i.p.) produced a significant mechanical antinociception in STOP null mice whereas it has no effect on wild-type mice. High concentration of naloxone (1 mg/kg) was pronociceptive for both mice strain, a lower concentration (0.1 mg/kg) was found to increase the mean mechanical nociceptive threshold only in the case of STOP null mice.
Conclusions: Together, our data show that STOP null mice displayed elevated levels of endogenous morphine, as well as an increase of morphine receptor affinity and density in brain. This was correlated with hypernociception and impaired pharmacological sensitivity to mu opioid receptor ligands.
Figures
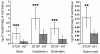

Similar articles
-
Prohormone convertase 2 (PC2) null mice have increased mu opioid receptor levels accompanied by altered morphine-induced antinociception, tolerance and dependence.Neuroscience. 2016 Aug 4;329:318-25. doi: 10.1016/j.neuroscience.2016.05.021. Epub 2016 May 18. Neuroscience. 2016. PMID: 27208618 Free PMC article.
-
Characterization of mechanical withdrawal responses and effects of mu-, delta- and kappa-opioid agonists in normal and mu-opioid receptor knockout mice.Brain Res. 1999 Mar 13;821(2):480-6. doi: 10.1016/s0006-8993(99)01060-4. Brain Res. 1999. PMID: 10064835
-
Ultra-low dose naloxone restores the antinociceptive effect of morphine in pertussis toxin-treated rats by reversing the coupling of mu-opioid receptors from Gs-protein to coupling to Gi-protein.Neuroscience. 2009 Dec 1;164(2):435-43. doi: 10.1016/j.neuroscience.2009.08.015. Epub 2009 Aug 12. Neuroscience. 2009. PMID: 19682558
-
Morphine can produce analgesia via spinal kappa opioid receptors in the absence of mu opioid receptors.Brain Res. 2006 Apr 14;1083(1):61-9. doi: 10.1016/j.brainres.2006.01.095. Epub 2006 Mar 10. Brain Res. 2006. PMID: 16530171
-
Emerging regulatory roles of opioid peptides, endogenous morphine, and opioid receptor subtypes in immunomodulatory processes: Metabolic, behavioral, and evolutionary perspectives.Immunol Lett. 2020 Nov;227:28-33. doi: 10.1016/j.imlet.2020.08.007. Epub 2020 Aug 19. Immunol Lett. 2020. PMID: 32827633 Review.
Cited by
-
Beyond Neuronal Microtubule Stabilization: MAP6 and CRMPS, Two Converging Stories.Front Mol Neurosci. 2021 May 5;14:665693. doi: 10.3389/fnmol.2021.665693. eCollection 2021. Front Mol Neurosci. 2021. PMID: 34025352 Free PMC article.
-
New horizons in schizophrenia treatment: autophagy protection is coupled with behavioral improvements in a mouse model of schizophrenia.Autophagy. 2014;10(12):2324-32. doi: 10.4161/15548627.2014.984274. Autophagy. 2014. PMID: 25484074 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

