Sleep state switching
- PMID: 21172606
- PMCID: PMC3026325
- DOI: 10.1016/j.neuron.2010.11.032
Sleep state switching
Abstract
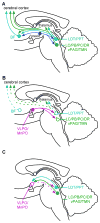
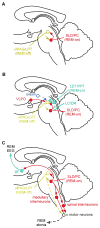
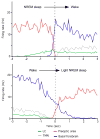
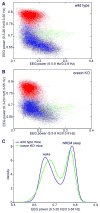
We take for granted the ability to fall asleep or to snap out of sleep into wakefulness, but these changes in behavioral state require specific switching mechanisms in the brain that allow well-defined state transitions. In this review, we examine the basic circuitry underlying the regulation of sleep and wakefulness and discuss a theoretical framework wherein the interactions between reciprocal neuronal circuits enable relatively rapid and complete state transitions. We also review how homeostatic, circadian, and allostatic drives help regulate sleep state switching and discuss how breakdown of the switching mechanism may contribute to sleep disorders such as narcolepsy.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
References
-
- Achermann P, Borbely AA. Mathematical models of sleep regulation. Front Biosci. 2003;8:s683–s693. - PubMed
-
- Adamantidis A, Salvert D, Goutagny R, Lakaye B, Gervasoni D, Grisar T, Luppi PH, Fort P. Sleep architecture of the melanin-concentrating hormone receptor 1-knockout mice. Eur J Neurosci. 2008;27:1793–1800. - PubMed
-
- Adametz JH. Rate of recovery of functioning in cats with rostral reticular lesions. J Neurosurg. 1959;16:85–98. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

