An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling
- PMID: 21172823
- PMCID: PMC3001412
- DOI: 10.1242/jcs.072686
An endophilin-dynamin complex promotes budding of clathrin-coated vesicles during synaptic vesicle recycling
Abstract
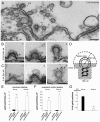
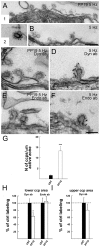
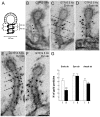
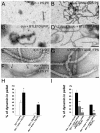
Clathrin-mediated vesicle recycling in synapses is maintained by a unique set of endocytic proteins and interactions. We show that endophilin localizes in the vesicle pool at rest and in spirals at the necks of clathrin-coated pits (CCPs) during activity in lamprey synapses. Endophilin and dynamin colocalize at the base of the clathrin coat. Protein spirals composed of these proteins on lipid tubes in vitro have a pitch similar to the one observed at necks of CCPs in living synapses, and lipid tubules are thinner than those formed by dynamin alone. Tubulation efficiency and the amount of dynamin recruited to lipid tubes are dramatically increased in the presence of endophilin. Blocking the interactions of the endophilin SH3 domain in situ reduces dynamin accumulation at the neck and prevents the formation of elongated necks observed in the presence of GTPγS. Therefore, endophilin recruits dynamin to a restricted part of the CCP neck, forming a complex, which promotes budding of new synaptic vesicles.
Figures


References
-
- Andersson F., Löw P., Brodin L. (2010). Selective perturbation of the BAR domain of endophilin impairs synaptic vesicle endocytosis. Synapse 64, 556-560 - PubMed
-
- Anggono V., Robinson P. J. (2007). Syndapin I and endophilin I bind overlapping proline-rich regions of dynamin I: role in synaptic vesicle endocytosis. J. Neurochem. 102, 931-943 - PubMed
-
- Conibear E. (2010). Converging views of endocytosis in yeast and mammals. Curr. Opin. Cell Biol. 22, 513-518 - PubMed
-
- Cremona O., Di Paolo G., Wenk M. R., Luthi A., Kim W. T., Takei K., Daniell L., Nemoto Y., Shears S. B., Flavell R. A., et al. (1999). Essential role of phosphoinositide metabolism in synaptic vesicle recycling. Cell 99, 179-188 - PubMed
-
- Dickman D. K., Horne J. A., Meinertzhagen I. A., Schwarz T. L. (2005). A slowed classical pathway rather than kiss-and-run mediates endocytosis at synapses lacking synaptojanin and endophilin. Cell 123, 521-533 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

