A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles
- PMID: 21173079
- PMCID: PMC3055552
- DOI: 10.1113/jphysiol.2010.201129
A functional role for the 'fibroblast-like cells' in gastrointestinal smooth muscles
Abstract
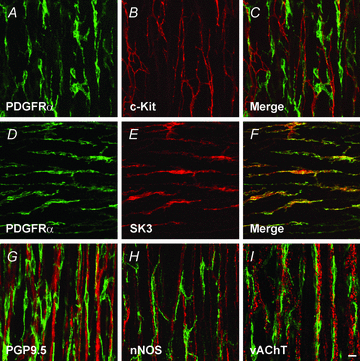
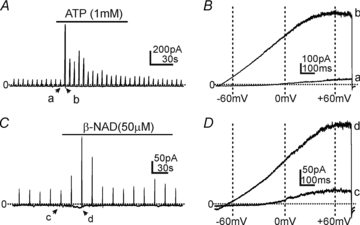
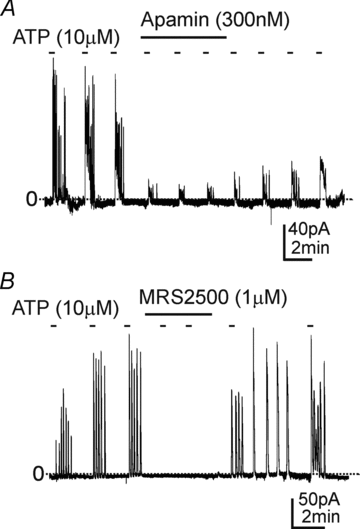
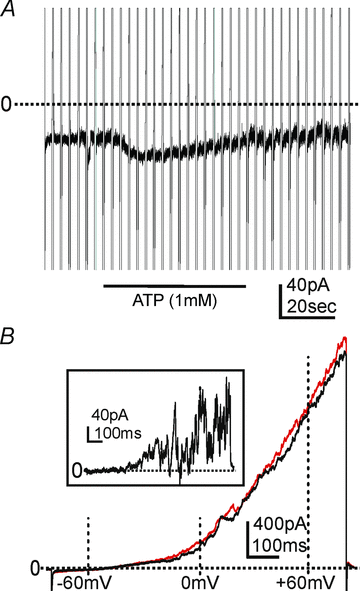
Smooth muscles, as in the gastrointestinal tract, are composed of several types of cells. Gastrointestinal muscles contain smooth muscle cells, enteric neurons, glial cells, immune cells, and various classes of interstitial cells. One type of interstitial cell, referred to as 'fibroblast-like cells' by morphologists, are common, but their function is unknown. These cells are found near the terminals of enteric motor neurons, suggesting they could have a role in generating neural responses that help control gastrointestinal movements. We used a novel mouse with bright green fluorescent protein expressed specifically in the fibroblast-like cells to help us identify these cells in the mixture of cells obtained when whole muscles are dispersed with enzymes. We isolated these cells and found they respond to a major class of inhibitory neurotransmitters - purines. We characterized these responses, and our results provide a new hypothesis about the role of fibroblast-like cells in smooth muscle tissues.
Figures





Comment in
-
Do 'fibroblast-like cells' intercede during enteric inhibitory motor neurotransmission in gastrointestinal smooth muscles?J Physiol. 2011 Feb 1;589(Pt 3):453-4. doi: 10.1113/jphysiol.2010.204016. J Physiol. 2011. PMID: 21285025 Free PMC article. No abstract available.
References
-
- Banks BE, Brown C, Burgess GM, Burnstock G, Claret M, Cocks TM, Jenkinson DH. Apamin blocks certain neurotransmitter-induced increases in potassium permeability. Nature. 1979;282:415–417. - PubMed
-
- Barfod ET, Moore AL, Lidofsky SD. Cloning and functional expression of a liver isoform of the small conductance Ca2+-activated K+ channel SK3. Am J Physiol Cell Physiol. 2001;280:C836–C842. - PubMed
-
- Bayguinov O, Hagen B, Bonev AD, Nelson MT, Sanders KM. Intracellular calcium events activated by ATP in murine colonic myocytes. Am J Physiol Cell Physiol. 2000;279:C126–C135. - PubMed
-
- Blatz AL, Magleby KL. Single apamin-blocked Ca-activated K+ channels of small conductance in cultured rat skeletal muscle. Nature. 1986;323:718–720. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

