Thymus-specific serine protease contributes to the diversification of the functional endogenous CD4 T cell receptor repertoire
- PMID: 21173102
- PMCID: PMC3023141
- DOI: 10.1084/jem.20100027
Thymus-specific serine protease contributes to the diversification of the functional endogenous CD4 T cell receptor repertoire
Abstract
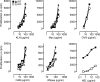
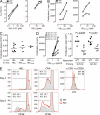
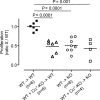
Thymus-specific serine protease (TSSP) is a novel protease that may contribute to the generation of the peptide repertoire presented by MHC class II molecules in the thymus. Although TSSP deficiency has no quantitative impact on the development of CD4 T cells expressing a polyclonal T cell receptor (TCR) repertoire, the development of CD4 T cells expressing the OTII and Marilyn transgenic TCRs is impaired in TSSP-deficient mice. In this study, we assess the role of TSSP in shaping the functional endogenous polyclonal CD4 T cell repertoire by analyzing the response of TSSP-deficient mice to several protein antigens (Ags). Although TSSP-deficient mice responded normally to most of the Ags tested, they responded poorly to hen egg lysozyme (HEL). The impaired CD4 T cell response of TSSP-deficient mice to HEL correlated with significant alteration of the dominant TCR-β chain repertoire expressed by HEL-specific CD4 T cells, suggesting that TSSP is necessary for the intrathymic development of cells expressing these TCRs. Thus, TSSP contributes to the diversification of the functional endogenous CD4 T cell TCR repertoire in the thymus.
Figures
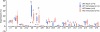
Similar articles
-
The T Cell Repertoire-Diversifying Enzyme TSSP Contributes to Thymic Selection of Diabetogenic CD4 T Cell Specificities Reactive to ChgA and IAPP Autoantigens.J Immunol. 2015 Sep 1;195(5):1964-73. doi: 10.4049/jimmunol.1401683. Epub 2015 Jul 24. J Immunol. 2015. PMID: 26209627
-
Thymus-specific serine protease regulates positive selection of a subset of CD4+ thymocytes.Eur J Immunol. 2009 Apr;39(4):956-64. doi: 10.1002/eji.200839175. Eur J Immunol. 2009. PMID: 19283781
-
Thymus-specific serine protease controls autoreactive CD4 T cell development and autoimmune diabetes in mice.J Clin Invest. 2011 May;121(5):1810-21. doi: 10.1172/JCI43314. Epub 2011 Apr 18. J Clin Invest. 2011. PMID: 21505262 Free PMC article.
-
Thymus-specific serine protease, a protease that shapes the CD4 T cell repertoire.Immunogenetics. 2019 Mar;71(3):223-232. doi: 10.1007/s00251-018-1078-y. Epub 2018 Sep 17. Immunogenetics. 2019. PMID: 30225612 Review.
-
The mechanisms shaping the repertoire of CD4+ Foxp3+ regulatory T cells.Immunology. 2018 Mar;153(3):290-296. doi: 10.1111/imm.12859. Epub 2017 Nov 22. Immunology. 2018. PMID: 29106696 Free PMC article. Review.
Cited by
-
Population and single-cell genomics reveal the Aire dependency, relief from Polycomb silencing, and distribution of self-antigen expression in thymic epithelia.Genome Res. 2014 Dec;24(12):1918-31. doi: 10.1101/gr.171645.113. Epub 2014 Sep 15. Genome Res. 2014. PMID: 25224068 Free PMC article.
-
Let the CAThepsin L out of the thymus.Nat Immunol. 2025 Jul;26(7):995-997. doi: 10.1038/s41590-025-02173-z. Nat Immunol. 2025. PMID: 40514417 No abstract available.
-
T-cell tolerance: central and peripheral.Cold Spring Harb Perspect Biol. 2012 Jun 1;4(6):a006957. doi: 10.1101/cshperspect.a006957. Cold Spring Harb Perspect Biol. 2012. PMID: 22661634 Free PMC article.
-
Revisiting thymic positive selection and the mature T cell repertoire for antigen.Immunity. 2014 Aug 21;41(2):181-90. doi: 10.1016/j.immuni.2014.07.007. Immunity. 2014. PMID: 25148022 Free PMC article.
-
Thymoproteasome and peptidic self.Immunogenetics. 2019 Mar;71(3):217-221. doi: 10.1007/s00251-018-1081-3. Epub 2018 Oct 15. Immunogenetics. 2019. PMID: 30324237 Review.
References
-
- Carrier A., Nguyen C., Victorero G., Granjeaud S., Rocha D., Bernard K., Miazek A., Ferrier P., Malissen M., Naquet P., et al. 1999. Differential gene expression in CD3epsilon- and RAG1-deficient thymuses: definition of a set of genes potentially involved in thymocyte maturation. Immunogenetics. 50:255–270 10.1007/s002510050601 - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

