A computational model predicts Xenopus meiotic spindle organization
- PMID: 21173114
- PMCID: PMC3010074
- DOI: 10.1083/jcb.201006076
A computational model predicts Xenopus meiotic spindle organization
Abstract
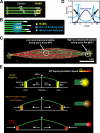
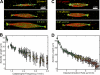
The metaphase spindle is a dynamic bipolar structure crucial for proper chromosome segregation, but how microtubules (MTs) are organized within the bipolar architecture remains controversial. To explore MT organization along the pole-to-pole axis, we simulated meiotic spindle assembly in two dimensions using dynamic MTs, a MT cross-linking force, and a kinesin-5-like motor. The bipolar structures that form consist of antiparallel fluxing MTs, but spindle pole formation requires the addition of a NuMA-like minus-end cross-linker and directed transport of MT depolymerization activity toward minus ends. Dynamic instability and minus-end depolymerization generate realistic MT lifetimes and a truncated exponential MT length distribution. Keeping the number of MTs in the simulation constant, we explored the influence of two different MT nucleation pathways on spindle organization. When nucleation occurs throughout the spindle, the simulation quantitatively reproduces features of meiotic spindles assembled in Xenopus egg extracts.
Figures


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

