Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation
- PMID: 21173118
- PMCID: PMC3062416
- DOI: 10.1182/blood-2010-10-313957
Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation
Abstract
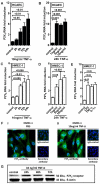
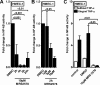
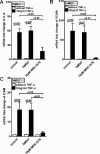
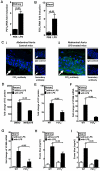
During a systemic inflammatory response endothelial-expressed surface molecules have been strongly implicated in orchestrating immune responses. Previous studies have shown enhanced extracellular nucleotide release during acute inflammatory conditions. Therefore, we hypothesized that endothelial nucleotide receptors could play a role in vascular inflammation. To address this hypothesis, we performed screening experiments and exposed human microvascular endothelia to inflammatory stimuli, followed by measurements of P2Y or P2X transcriptional responses. These studies showed a selective induction of the P2Y(6) receptor (> 4-fold at 24 hours). Moreover, studies that used real-time reverse transcription-polymerase chain reaction, Western blot analysis, or immunofluorescence confirmed time- and dose-dependent induction of P2Y(6) with tumor necrosis factor α or Lipopolysaccharide (LPS) stimulation in vitro and in vivo. Studies that used MRS 2578 as P2Y(6) receptor antagonist showed attenuated nuclear factor κB reporter activity and proinflammatory gene expression in human microvascular endothelial cells in vitro. Moreover, pharmacologic or genetic in vivo studies showed attenuated inflammatory responses in P2Y(6)(-/-) mice or after P2Y(6) antagonist treatment during LPS-induced vascular inflammation. These studies show an important contribution of P2Y(6) signaling in enhancing vascular inflammation during systemic LPS challenge and implicate the P2Y(6) receptor as a therapeutic target during systemic inflammatory responses.
Figures

Comment in
-
P2Y6 and vascular inflammation.Blood. 2011 Feb 24;117(8):2304-5. doi: 10.1182/blood-2011-01-327973. Blood. 2011. PMID: 21350062
References
-
- Eltzschig HK, Collard CD. Vascular ischaemia and reperfusion injury. Br Med Bull. 2004;70(1):71–86. - PubMed
-
- Eckle T, Koeppen M, Eltzschig HK. Role of extracellular adenosine in acute lung injury. Physiology (Bethesda) 2009;24(5):298–306. - PubMed
-
- Eltzschig HK, Eckle T, Mager A, et al. ATP release from activated neutrophils occurs via connexin 43 and modulates adenosine-dependent endothelial cell function. Circ Res. 2006;99(10):1100–1108. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

