ATP induces conformational changes in the carboxyl-terminal region of ClC-5
- PMID: 21173145
- PMCID: PMC3057859
- DOI: 10.1074/jbc.M110.175877
ATP induces conformational changes in the carboxyl-terminal region of ClC-5
Abstract
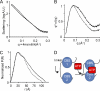
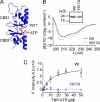
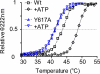
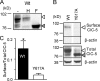
ATP binding enhances the activity of ClC-5, the transporter mutated in Dent disease, a disease affecting the renal proximal tubule. Previously, the ATP binding site was revealed in x-ray crystal structures of the cytoplasmic region of this membrane protein. Disruption of this site by mutagenesis (Y617A-ClC-5) reduced the functional expression and ATP-dependent regulation of the full-length transporter in Xenopus oocytes. However, insight into the conformational changes underlying ATP-dependent regulation is lacking. Here, we show that ATP binding induces a change in protein conformation. Specifically, small angle x-ray scattering experiments indicate that ATP binding promotes a clamp-like closure of the isolated ClC-5 carboxyl-terminal region. Limited proteolysis studies show that ATP binding induces conformational compaction of the carboxyl-terminal region in the intact membrane protein as well. In the context of fibroblasts and proximal tubule epithelial cells, disruption of the ATP binding site in full-length ClC-5 (Y617A-ClC-5) led to a defect in processing and trafficking out of the endoplasmic reticulum. These latter findings account for the decrease in functional expression previously reported for this ATP-binding mutant and prompt future study of a model whereby conformational compaction caused by ATP binding promotes biosynthetic maturation.
Figures



Similar articles
-
Conformational defects underlie proteasomal degradation of Dent's disease-causing mutants of ClC-5.Biochem J. 2013 Jun 15;452(3):391-400. doi: 10.1042/BJ20121848. Biochem J. 2013. PMID: 23566014
-
Nucleotide recognition by the cytoplasmic domain of the human chloride transporter ClC-5.Nat Struct Mol Biol. 2007 Jan;14(1):60-7. doi: 10.1038/nsmb1188. Epub 2006 Dec 31. Nat Struct Mol Biol. 2007. PMID: 17195847
-
Diversity of functional alterations of the ClC-5 exchanger in the region of the proton glutamate in patients with Dent disease 1.Hum Mutat. 2021 May;42(5):537-550. doi: 10.1002/humu.24184. Epub 2021 Mar 1. Hum Mutat. 2021. PMID: 33600050
-
Heterogeneity in the processing of CLCN5 mutants related to Dent disease.Hum Mutat. 2011 Apr;32(4):476-83. doi: 10.1002/humu.21467. Hum Mutat. 2011. PMID: 21305656
-
Mutation Update of the CLCN5 Gene Responsible for Dent Disease 1.Hum Mutat. 2015 Aug;36(8):743-52. doi: 10.1002/humu.22804. Epub 2015 Jun 11. Hum Mutat. 2015. PMID: 25907713 Review.
Cited by
-
CLC anion channel regulatory phosphorylation and conserved signal transduction domains.Biophys J. 2012 Oct 17;103(8):1706-18. doi: 10.1016/j.bpj.2012.09.001. Epub 2012 Oct 16. Biophys J. 2012. PMID: 23083714 Free PMC article.
-
Regulation of ClC-2 gating by intracellular ATP.Pflugers Arch. 2013 Oct;465(10):1423-37. doi: 10.1007/s00424-013-1286-0. Epub 2013 May 1. Pflugers Arch. 2013. PMID: 23632988 Free PMC article.
-
Making a Dent in Dent Disease.Function (Oxf). 2020;1(2):zqaa017. doi: 10.1093/function/zqaa017. Epub 2020 Sep 11. Function (Oxf). 2020. PMID: 33015630 Free PMC article. Review.
-
Intracellular β-nicotinamide adenine dinucleotide inhibits the skeletal muscle ClC-1 chloride channel.J Biol Chem. 2012 Jul 27;287(31):25808-20. doi: 10.1074/jbc.M111.327551. Epub 2012 Jun 11. J Biol Chem. 2012. PMID: 22689570 Free PMC article.
-
Purinergic signalling in the kidney in health and disease.Purinergic Signal. 2014 Mar;10(1):71-101. doi: 10.1007/s11302-013-9400-5. Epub 2013 Nov 22. Purinergic Signal. 2014. PMID: 24265071 Free PMC article. Review.
References
-
- Lloyd S. E., Pearce S. H., Fisher S. E., Steinmeyer K., Schwappach B., Scheinman S. J., Harding B., Bolino A., Devoto M., Goodyer P., Rigden S. P., Wrong O., Jentsch T. J., Craig I. W., Thakker R. V. (1996) Nature 379, 445–449 - PubMed
-
- Lloyd S. E., Gunther W., Pearce S. H., Thomson A., Bianchi M. L., Bosio M., Craig I. W., Fisher S. E., Scheinman S. J., Wrong O., Jentsch T. J., Thakker R. V. (1997) Hum. Mol. Genet. 6, 1233–1239 - PubMed
-
- Wrong O. M., Norden A. G., Feest T. G. (1994) Q. J. Med. 87, 473–493 - PubMed
-
- Scheel O., Zdebik A. A., Lourdel S., Jentsch T. J. (2005) Nature 436, 424–427 - PubMed
-
- Picollo A., Pusch M. (2005) Nature 436, 420–423 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

