ATP modulates interaction of syntaxin-1A with sulfonylurea receptor 1 to regulate pancreatic beta-cell KATP channels
- PMID: 21173146
- PMCID: PMC3037700
- DOI: 10.1074/jbc.M109.089607
ATP modulates interaction of syntaxin-1A with sulfonylurea receptor 1 to regulate pancreatic beta-cell KATP channels
Abstract
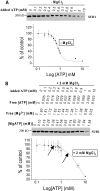
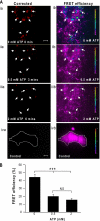
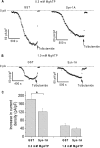
ATP-sensitive potassium (K(ATP)) channels are regulated by a variety of cytosolic factors (adenine nucleotides, Mg(2+), phospholipids, and pH). We previously reported that K(ATP) channels are also regulated by endogenous membrane-bound SNARE protein syntaxin-1A (Syn-1A), which binds both nucleotide-binding folds of sulfonylurea receptor (SUR)1 and 2A, causing inhibition of K(ATP) channel activity in pancreatic islet β-cells and cardiac myocytes, respectively. In this study, we show that ATP dose-dependently inhibits Syn-1A binding to SUR1 at physiological concentrations, with the addition of Mg(2+) causing a decrease in the ATP-induced inhibitory effect. This ATP disruption of Syn-1A binding to SUR1 was confirmed by FRET analysis in living HEK293 cells. Electrophysiological studies in pancreatic β-cells demonstrated that reduced ATP concentrations increased K(ATP) channel sensitivity to Syn-1A inhibition. Depletion of endogenous Syn-1A in insulinoma cells by botulinum neurotoxin C1 proteolysis followed by rescue with exogenous Syn-1A showed that Syn-1A modulates K(ATP) channel sensitivity to ATP. Thus, our data indicate that although both ATP and Syn-1A independently inhibit β-cell K(ATP) channel gating, they could also influence the sensitivity of K(ATP) channels to each other. These findings provide new insight into an alternate mechanism by which ATP regulates pancreatic β-cell K(ATP) channel activity, not only by its direct actions on Kir6.2 pore subunit, but also via ATP modulation of Syn-1A binding to SUR1.
Figures

Similar articles
-
Syntaxin-1A interacts with distinct domains within nucleotide-binding folds of sulfonylurea receptor 1 to inhibit beta-cell ATP-sensitive potassium channels.J Biol Chem. 2011 Jul 1;286(26):23308-18. doi: 10.1074/jbc.M111.217950. Epub 2011 May 3. J Biol Chem. 2011. PMID: 21540180 Free PMC article.
-
Phosphatidylinositol 4,5-biphosphate (PIP2) modulates interaction of syntaxin-1A with sulfonylurea receptor 1 to regulate pancreatic β-cell ATP-sensitive potassium channels.J Biol Chem. 2014 Feb 28;289(9):6028-40. doi: 10.1074/jbc.M113.511808. Epub 2014 Jan 15. J Biol Chem. 2014. PMID: 24429282 Free PMC article.
-
H3 domain of syntaxin 1A inhibits KATP channels by its actions on the sulfonylurea receptor 1 nucleotide-binding folds-1 and -2.J Biol Chem. 2004 Dec 17;279(51):53259-65. doi: 10.1074/jbc.M410171200. Epub 2004 Oct 13. J Biol Chem. 2004. PMID: 15485808
-
The structure and function of the ATP-sensitive K+ channel in insulin-secreting pancreatic beta-cells.J Mol Endocrinol. 1999 Apr;22(2):113-23. doi: 10.1677/jme.0.0220113. J Mol Endocrinol. 1999. PMID: 10194514 Review.
-
Comparative aspects of the function and mechanism of SUR1 and MDR1 proteins.Biochim Biophys Acta. 1999 Dec 6;1461(2):305-13. doi: 10.1016/s0005-2736(99)00157-1. Biochim Biophys Acta. 1999. PMID: 10581363 Review.
Cited by
-
Kv2.1 clusters on β-cell plasma membrane act as reservoirs that replenish pools of newcomer insulin granule through their interaction with syntaxin-3.J Biol Chem. 2018 May 4;293(18):6893-6904. doi: 10.1074/jbc.RA118.002703. Epub 2018 Mar 16. J Biol Chem. 2018. PMID: 29549124 Free PMC article.
-
Current understanding of K ATP channels in neonatal diseases: focus on insulin secretion disorders.Acta Pharmacol Sin. 2011 Jun;32(6):765-80. doi: 10.1038/aps.2011.57. Epub 2011 May 23. Acta Pharmacol Sin. 2011. PMID: 21602835 Free PMC article. Review.
-
Stromal Interaction Molecule 1 (STIM1) Regulates ATP-sensitive Potassium (KATP) and Store-operated Ca2+ Channels in MIN6 β-Cells.J Biol Chem. 2017 Feb 10;292(6):2266-2277. doi: 10.1074/jbc.M116.767681. Epub 2016 Dec 21. J Biol Chem. 2017. PMID: 28003364 Free PMC article.
-
K(ATP) channels and islet hormone secretion: new insights and controversies.Nat Rev Endocrinol. 2013 Nov;9(11):660-9. doi: 10.1038/nrendo.2013.166. Epub 2013 Sep 17. Nat Rev Endocrinol. 2013. PMID: 24042324 Free PMC article. Review.
-
Blocking Kir6.2 channels with SpTx1 potentiates glucose-stimulated insulin secretion from murine pancreatic β cells and lowers blood glucose in diabetic mice.Elife. 2022 Feb 25;11:e77026. doi: 10.7554/eLife.77026. Elife. 2022. PMID: 35212627 Free PMC article.
References
-
- Inagaki N., Tsuura Y., Namba N., Masuda K., Gonoi T., Horie M., Seino Y., Mizuta M., Seino S. (1995) J. Biol. Chem. 270, 5691–5694 - PubMed
-
- Tarasov A. I., Girard C. A., Ashcroft F. M. (2006) Diabetes 55, 2446–2454 - PubMed
-
- Clement J. P., 4th, Kunjilwar K., Gonzalez G., Schwanstecher M., Panten U., Aguilar-Bryan L., Bryan J. (1997) Neuron 18, 827–838 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

