A Na+-translocating pyrophosphatase in the acetogenic bacterium Acetobacterium woodii
- PMID: 21173152
- PMCID: PMC3057867
- DOI: 10.1074/jbc.M110.192823
A Na+-translocating pyrophosphatase in the acetogenic bacterium Acetobacterium woodii
Abstract
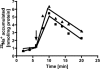
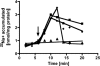
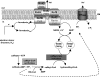
The anaerobic acetogenic bacterium Acetobacterium woodii employs a novel type of Na(+)-motive anaerobic respiration, caffeate respiration. However, this respiration is at the thermodynamic limit of energy conservation, and even worse, in the first step, caffeate is activated by caffeyl-CoA synthetase, which hydrolyzes ATP to AMP and pyrophosphate. Here, we have addressed whether or not the energy stored in the anhydride bond of pyrophosphate is conserved by A. woodii. Inverted membrane vesicles of A. woodii have a membrane-bound pyrophosphatase that catalyzes pyrophosphate hydrolysis at a rate of 70-120 milliunits/mg of protein. Pyrophosphatase activity was dependent on the divalent cation Mg(2+). In addition, activity was strictly dependent on Na(+) with a K(m) of 1.1 mM. Hydrolysis of pyrophosphate was accompanied by (22)Na(+) transport into the lumen of the inverted membrane vesicles. Inhibitor studies revealed that (22)Na(+) transport was primary and electrogenic. Next to the Na(+)-motive ferredoxin:NAD(+) oxidoreductase (Fno or Rnf), the Na(+)-pyrophosphatase is the second primary Na(+)-translocating enzyme in A. woodii.
Figures
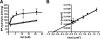
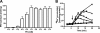
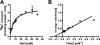
References
-
- Müller V., Imkamp F., Rauwolf A., Küsel K., Drake H. L. (2004) in Strict and Facultative Anaerobes: Medical and Environmental Aspects (Nakano M. M., Zuber P. eds) pp. 251–281, Horizon Biosciences, Norfolk
-
- Schmidt S., Biegel E., Müller V. (2009) Biochim. Biophys. Acta 1787, 691–696 - PubMed
-
- Müller V., Aufurth S., Rahlfs S. (2001) Biochim. Biophys. Acta 1505, 108–120 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

