Bax activation by engagement with, then release from, the BH3 binding site of Bcl-xL
- PMID: 21173168
- PMCID: PMC3028639
- DOI: 10.1128/MCB.00161-10
Bax activation by engagement with, then release from, the BH3 binding site of Bcl-xL
Abstract
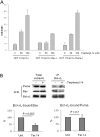
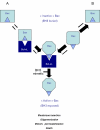
Bcl-2 homologues (such as Bcl-x(L)) promote survival in part through sequestration of "activator" BH3-only proteins (such as Puma), preventing them from directly activating Bax. It is thus assumed that inhibition of interactions between activators and Bcl-x(L) is a prerequisite for small molecules to antagonize Bcl-x(L) and induce cell death. The biological properties, described here of a terphenyl-based alpha-helical peptidomimetic inhibitor of Bcl-x(L) attest that displacement of Bax from Bcl-x(L) is also critical. Terphenyl 14 triggers Bax-dependent but Puma-independent cell death, disrupting Bax/Bcl-x(L) interactions without affecting Puma/Bcl-x(L) interactions. In cell-free assays, binding of inactive Bax to Bcl-x(L), followed by its displacement from Bcl-x(L) by terphenyl 14, produces mitochondrially permeabilizing Bax molecules. Moreover, the peptidomimetic kills yeast cells that express Bax and Bcl-x(L), and it uses Bax-binding Bcl-x(L) to induce mammalian cell death. Likewise, ectopic expression of Bax in yeast and mammalian cells enhances sensitivity to another Bcl-x(L) inhibitor, ABT-737, when Bcl-x(L) is present. Thus, the interaction of Bcl-x(L) with Bax paradoxically primes Bax at the same time it keeps Bax activity in check, and displacement of Bax from Bcl-x(L) triggers an apoptotic signal by itself. This mechanism might contribute to the clinical efficiency of Bcl-x(L) inhibitors.
Figures






Similar articles
-
Bax/Bak activation in the absence of Bid, Bim, Puma, and p53.Cell Death Dis. 2016 Jun 16;7(6):e2266. doi: 10.1038/cddis.2016.167. Cell Death Dis. 2016. PMID: 27310874 Free PMC article.
-
Bim upregulation by histone deacetylase inhibitors mediates interactions with the Bcl-2 antagonist ABT-737: evidence for distinct roles for Bcl-2, Bcl-xL, and Mcl-1.Mol Cell Biol. 2009 Dec;29(23):6149-69. doi: 10.1128/MCB.01481-08. Epub 2009 Oct 5. Mol Cell Biol. 2009. PMID: 19805519 Free PMC article.
-
Bax activation by the BH3-only protein Puma promotes cell dependence on antiapoptotic Bcl-2 family members.J Cell Biol. 2009 Apr 20;185(2):279-90. doi: 10.1083/jcb.200809153. J Cell Biol. 2009. PMID: 19380879 Free PMC article.
-
A brewing understanding of the regulation of Bax function by Bcl-xL and Bcl-2.Mech Ageing Dev. 2017 Jan;161(Pt B):201-210. doi: 10.1016/j.mad.2016.04.007. Epub 2016 Apr 23. Mech Ageing Dev. 2017. PMID: 27112371 Review.
-
Small molecule inhibition of the Bcl-X(L)-BH3 protein-protein interaction: proof-of-concept of an in vivo chemopotentiator ABT-737.Curr Top Med Chem. 2007;7(10):961-5. doi: 10.2174/156802607780906843. Curr Top Med Chem. 2007. PMID: 17508927 Review.
Cited by
-
Bcl-xL controls a switch between cell death modes during mitotic arrest.Cell Death Dis. 2014 Jun 12;5(6):e1291. doi: 10.1038/cddis.2014.251. Cell Death Dis. 2014. PMID: 24922075 Free PMC article.
-
Targeting of BCL-2 Family Members during Anticancer Treatment: A Necessary Compromise between Individual Cell and Ecosystemic Responses?Biomolecules. 2020 Jul 25;10(8):1109. doi: 10.3390/biom10081109. Biomolecules. 2020. PMID: 32722518 Free PMC article. Review.
-
TOM20-mediated transfer of Bcl2 from ER to MAM and mitochondria upon induction of apoptosis.Cell Death Dis. 2021 Feb 15;12(2):182. doi: 10.1038/s41419-021-03471-8. Cell Death Dis. 2021. PMID: 33589622 Free PMC article.
-
Improving the therapeutic potential of endostatin by fusing it with the BAX BH3 death domain.Cell Death Dis. 2014 Aug 14;5(8):e1371. doi: 10.1038/cddis.2014.309. Cell Death Dis. 2014. PMID: 25118931 Free PMC article.
-
Bcl-2 Family Members and the Mitochondrial Import Machineries: The Roads to Death.Biomolecules. 2022 Jan 19;12(2):162. doi: 10.3390/biom12020162. Biomolecules. 2022. PMID: 35204663 Free PMC article. Review.
References
-
- Amundson, S. A., et al. 2000. An informatics approach identifying markers of chemosensitivity in human cancer cell lines. Cancer Res. 60:6101-6110. - PubMed
-
- Arokium, H., N. Camougrand, F. M. Vallette, and S. Manon. 2004. Studies of the interaction of substituted mutants of BAX with yeast mitochondria reveal that the C-terminal hydrophobic alpha-helix is a second ART sequence and plays a role in the interaction with anti-apoptotic BCL-xL. J. Biol. Chem. 279:52566-52573. - PubMed
-
- Cartron, P. F., et al. 2004. The first alpha helix of Bax plays a necessary role in its ligand-induced activation by the BH3-only proteins Bid and PUMA. Mol. Cell 16:807-818. - PubMed
-
- Cartron, P. F., L. Oliver, E. Mayat, K. Meflah, and F. M. Vallette. 2004. Impact of pH on Bax alpha conformation, oligomerisation and mitochondrial integration. FEBS Lett. 578:41-46. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
