Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease
- PMID: 21173186
- PMCID: PMC3067123
- DOI: 10.1128/AAC.01402-10
Systematic review and meta-analysis of the effectiveness and safety of tigecycline for treatment of infectious disease
Abstract
The aim of this study was to compare the efficacy and safety of tigecycline, a newly developed glycylcycline antibiotic, with those of empirical antibiotic regimens which have been reported to possess good efficacy for complicated skin and skin structure infections (cSSSIs), complicated intra-abdominal infections (cIAIs), community-acquired pneumonia (CAP), and other infections caused by methicillin-resistant Staphylococcus aureus (MRSA) or vancomycin-resistant Enterococcus (VRE). A meta-analysis of randomized controlled trials (RCTs) identified in PubMed, the Cochrane Library, and Embase was performed. Eight RCTs involving 4,651 patients were included in the meta-analysis. Compared with therapy with empirical antibiotic regimens, tigecycline monotherapy was associated with similar clinical treatment success rates (for the clinically evaluable [CE] population, odds ratio [OR] = 0.92, 95% confidence interval [CI] = 0.76 to 1.12, P = 0.42; for the clinical modified intent-to-treat [c-mITT] population, OR = 0.86, 95% CI = 0.74 to 1.01, P = 0.06) and similar microbiological treatment success rates (for the microbiologically evaluable [ME] population, OR = 0.86, 95% CI = 0.69 to 1.07, P = 0.19). The incidence of adverse events in the tigecycline group was significantly higher than that in the other therapy groups with a statistical margin (for the modified intent-to-treat [mITT] population, OR = 1.33, 95% CI = 1.17 to 1.52, P < 0.0001), especially in the digestive system (mITT population, OR = 2.41, 95% CI = 1.67 to 3.46, P < 0.00001). No difference regarding all-cause mortality and drug-related mortality between tigecycline and the other regimens was found, although numerically higher mortality was found in the tigecycline group. This meta-analysis provides evidence that tigecycline monotherapy may be used as effectively as the comparison therapy for cSSSI, cIAIs, CAP, and infections caused by MRSA/VRE. However, because of the high risk of mortality, AEs, and emergence of resistant isolates, prudence with the clinical use of tigecycline monotherapy in infections is required.
Figures


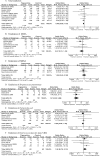
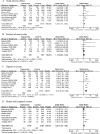


Comment in
-
Clarification to the systematic review and meta-analysis involving tigecycline.Antimicrob Agents Chemother. 2011 Oct;55(10):4941. doi: 10.1128/AAC.00512-11. Antimicrob Agents Chemother. 2011. PMID: 21921119 Free PMC article. No abstract available.
References
-
- Anthony, K. B., et al. 2008. Clinical and microbiological outcomes of serious infections with multidrug-resistant gram-negative organisms treated with tigecycline. Clin. Infect. Dis. 46:567-570. - PubMed
-
- Babinchak, T., et al. 2005. The efficacy and safety of tigecycline for the treatment of complicated intra-abdominal infections: analysis of pooled clinical trial data. Clin. Infect. Dis. 41(Suppl. 5):S354-S367. - PubMed
-
- Bergallo, C., et al. 2009. Safety and efficacy of intravenous tigecycline in treatment of community-acquired pneumonia: results from a double-blind randomized phase 3 comparison study with levofloxacin. Diagn. Microbiol. Infect. Dis. 63:52-61. - PubMed
-
- Bhattacharya, M., A. Parakh, and M. Narang. 2009. Tigecycline. J. Postgrad. Med. 55:65-68. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

