Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria
- PMID: 21173190
- PMCID: PMC3035681
- DOI: 10.1194/jlr.M007575
Enzymatic activities of the human AGPAT isoform 3 and isoform 5: localization of AGPAT5 to mitochondria
Abstract
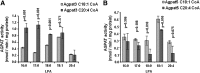
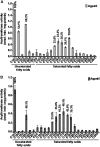
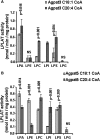
The enzyme 1-acylglycerol-3-phosphate-O-acyltransferase (AGPAT) converts lysophosphatidic acid (LPA) to phosphatidic acid (PA). In this study, we show enzymatic properties, tissue distribution, and subcellular localization of human AGPAT3 and AGPAT5. In cells overexpressing these isoforms, the proteins were detected in the nuclear envelope and the endoplasmic reticulum. AGPAT5-GFP fusion protein was localized in the mitochondria of both Chinese hamster ovary and human epithelial cervical cancer cells. Using lysates of AD293 cells infected with AGPAT3 and AGPAT5 recombinant adenovirus, we show that AGPAT3 and AGPAT5 proteins have AGPAT activity. Both the isoforms have similar apparent V(max) of 6.35 and 2.42 nmol/min/mg protein, respectively, for similar LPA. The difference between the two isoforms is in their use of additional lysophospholipids. AGPAT3 shows significant esterification of lysophosphatidylinositol (LPI) in the presence of C20:4 fatty acid, whereas AGPAT5 demonstrates significant acyltransferase activity toward lysophosphatidylethanolamine (LPE) in the presence of C18:1 fatty acid. The AGPAT3 mRNA is ubiquitously expressed in human tissues with several-fold differences in the expression pattern compared with the closely related AGPAT4. In summary, we show that in the presence of different fatty acids, AGPAT3 and AGPAT5 prefer different lysophospholipids as acyl acceptors. More importantly, localization of overexpressed AGPAT5 (this study) as well as GPAT1 and 2 (previous studies) in mitochondria supports the idea that the mitochondria might be capable of synthesizing some of their own glycerophospholipids.
Figures




Similar articles
-
The Structure and Function of Acylglycerophosphate Acyltransferase 4/ Lysophosphatidic Acid Acyltransferase Delta (AGPAT4/LPAATδ).Front Cell Dev Biol. 2019 Aug 2;7:147. doi: 10.3389/fcell.2019.00147. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31428612 Free PMC article. Review.
-
Functional characterization of the human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 10/glycerol-3-phosphate acyltransferase isoform 3.J Mol Endocrinol. 2009 Jun;42(6):469-78. doi: 10.1677/JME-09-0010. Epub 2009 Mar 24. J Mol Endocrinol. 2009. PMID: 19318427 Free PMC article.
-
Functional characterization of human 1-acylglycerol-3-phosphate-O-acyltransferase isoform 9: cloning, tissue distribution, gene structure, and enzymatic activity.J Endocrinol. 2007 Jun;193(3):445-57. doi: 10.1677/JOE-07-0027. J Endocrinol. 2007. PMID: 17535882
-
Functional characterization of human 1-acylglycerol-3-phosphate acyltransferase isoform 8: cloning, tissue distribution, gene structure, and enzymatic activity.Arch Biochem Biophys. 2006 May 15;449(1-2):64-76. doi: 10.1016/j.abb.2006.03.014. Epub 2006 Mar 29. Arch Biochem Biophys. 2006. PMID: 16620771
-
Mitochondrial acyltransferases and glycerophospholipid metabolism.Biochim Biophys Acta Mol Cell Biol Lipids. 2017 Jan;1862(1):49-55. doi: 10.1016/j.bbalip.2016.06.023. Epub 2016 Jul 1. Biochim Biophys Acta Mol Cell Biol Lipids. 2017. PMID: 27377347 Review.
Cited by
-
Human 1-acylglycerol-3-phosphate O-acyltransferase isoforms 1 and 2: biochemical characterization and inability to rescue hepatic steatosis in Agpat2(-/-) gene lipodystrophic mice.J Biol Chem. 2011 Oct 28;286(43):37676-91. doi: 10.1074/jbc.M111.250449. Epub 2011 Aug 27. J Biol Chem. 2011. PMID: 21873652 Free PMC article.
-
Probing the Global Cellular Responses to Lipotoxicity Caused by Saturated Fatty Acids.Mol Cell. 2019 Apr 4;74(1):32-44.e8. doi: 10.1016/j.molcel.2019.01.036. Epub 2019 Mar 4. Mol Cell. 2019. PMID: 30846318 Free PMC article.
-
Identification and functional prediction of lncRNAs associated with intramuscular lipid deposition in Guangling donkeys.Front Vet Sci. 2024 Jul 5;11:1410109. doi: 10.3389/fvets.2024.1410109. eCollection 2024. Front Vet Sci. 2024. PMID: 39036793 Free PMC article.
-
The Structure and Function of Acylglycerophosphate Acyltransferase 4/ Lysophosphatidic Acid Acyltransferase Delta (AGPAT4/LPAATδ).Front Cell Dev Biol. 2019 Aug 2;7:147. doi: 10.3389/fcell.2019.00147. eCollection 2019. Front Cell Dev Biol. 2019. PMID: 31428612 Free PMC article. Review.
-
Phospholipid Acyltransferases: Characterization and Involvement of the Enzymes in Metabolic and Cancer Diseases.Cancers (Basel). 2024 May 31;16(11):2115. doi: 10.3390/cancers16112115. Cancers (Basel). 2024. PMID: 38893234 Free PMC article. Review.
References
-
- Merkl I., Lands W. E. 1963. Metabolism of glycerolipids. IV. Synthesis of phosphatidylethanolamine. J. Biol. Chem. 238: 905–906. - PubMed
-
- Agarwal A. K., Garg A. 2003. Congenital generalized lipodystrophy: significance of triglyceride biosynthetic pathways. Trends Endocrinol. Metab. 14: 214–221. - PubMed
-
- Coleman R. A., Lee D. P. 2004. Enzymes of triacylglycerol synthesis and their regulation. Prog. Lipid Res. 43: 134–176. - PubMed
-
- Eberhardt C., Gray P. W., Tjoelker L. W. 1997. Human lysophosphatidic acid acyltransferase. cDNA cloning, expression, and localization to chromosome 9q34.3. J. Biol. Chem. 272: 20299–20305. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

