Chimpanzees as an animal model for human norovirus infection and vaccine development
- PMID: 21173246
- PMCID: PMC3017165
- DOI: 10.1073/pnas.1014577107
Chimpanzees as an animal model for human norovirus infection and vaccine development
Abstract
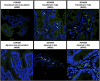
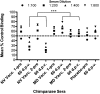
Noroviruses are global agents of acute gastroenteritis, but the development of control strategies has been hampered by the absence of a robust animal model. Studies in chimpanzees have played a key role in the characterization of several fastidious hepatitis viruses, and we investigated the feasibility of such studies for the noroviruses. Seronegative chimpanzees inoculated i.v. with the human norovirus strain Norwalk virus (NV) did not show clinical signs of gastroenteritis, but the onset and duration of virus shedding in stool and serum antibody responses were similar to that observed in humans. NV RNA was detected in intestinal and liver biopsies concurrent with the detection of viral shedding in stool, and NV antigen expression was observed in cells of the small intestinal lamina propria. Two infected chimpanzees rechallenged 4, 10, or 24 mo later with NV were resistant to reinfection, and the presence of NV-specific serum antibodies correlated with protection. We evaluated the immunogenicity and efficacy of virus-like particles (VLPs) derived from NV (genogroup I, GI) and MD145 (genogroup II, GII) noroviruses as vaccines. Chimpanzees vaccinated intramuscularly with GI VLPs were protected from NV infection when challenged 2 and 18 mo after vaccination, whereas chimpanzees that received GII VLPs vaccine or a placebo were not. This study establishes the chimpanzee as a viable animal model for the study of norovirus replication and immunity, and shows that NV VLP vaccines could induce protective homologous immunity even after extended periods of time.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
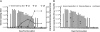
References
-
- Anonymous; Centers for Disease Control and Prevention (CDC) Surveillance for foodborne disease outbreaks—United States, 2006. MMWR Morb Mortal Wkly Rep. 2009;58:609–615. - PubMed
-
- Tacket CO, Sztein MB, Losonsky GA, Wasserman SS, Estes MK. Humoral, mucosal, and cellular immune responses to oral Norwalk virus-like particles in volunteers. Clin Immunol. 2003;108:241–247. - PubMed
-
- Dolin R, et al. Biological properties of Norwalk agent of acute infectious nonbacterial gastroenteritis. Proc Soc Exp Biol Med. 1972;140:578–583. - PubMed
-
- Dolin R, et al. Transmission of acute infectious nonbacterial gastroenteritis to volunteers by oral administration of stool filtrates. J Infect Dis. 1971;123:307–312. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

