Global declines in oceanic nitrification rates as a consequence of ocean acidification
- PMID: 21173255
- PMCID: PMC3017153
- DOI: 10.1073/pnas.1011053108
Global declines in oceanic nitrification rates as a consequence of ocean acidification
Abstract
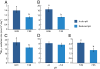
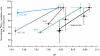
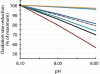
Ocean acidification produced by dissolution of anthropogenic carbon dioxide (CO(2)) emissions in seawater has profound consequences for marine ecology and biogeochemistry. The oceans have absorbed one-third of CO(2) emissions over the past two centuries, altering ocean chemistry, reducing seawater pH, and affecting marine animals and phytoplankton in multiple ways. Microbially mediated ocean biogeochemical processes will be pivotal in determining how the earth system responds to global environmental change; however, how they may be altered by ocean acidification is largely unknown. We show here that microbial nitrification rates decreased in every instance when pH was experimentally reduced (by 0.05-0.14) at multiple locations in the Atlantic and Pacific Oceans. Nitrification is a central process in the nitrogen cycle that produces both the greenhouse gas nitrous oxide and oxidized forms of nitrogen used by phytoplankton and other microorganisms in the sea; at the Bermuda Atlantic Time Series and Hawaii Ocean Time-series sites, experimental acidification decreased ammonia oxidation rates by 38% and 36%. Ammonia oxidation rates were also strongly and inversely correlated with pH along a gradient produced in the oligotrophic Sargasso Sea (r(2) = 0.87, P < 0.05). Across all experiments, rates declined by 8-38% in low pH treatments, and the greatest absolute decrease occurred where rates were highest off the California coast. Collectively our results suggest that ocean acidification could reduce nitrification rates by 3-44% within the next few decades, affecting oceanic nitrous oxide production, reducing supplies of oxidized nitrogen in the upper layers of the ocean, and fundamentally altering nitrogen cycling in the sea.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Solomon S, et al. In: Climate Change 2007: The Physical Science Basis. Contribution of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. Solomon S, et al., editors. Cambridge, and New York, NY: Cambridge Univ Press; 2007. pp. 19–92.
-
- Caldeira K, Wickett ME. Oceanography: Anthropogenic carbon and ocean pH. Nature. 2003;425:365. - PubMed
-
- Sabine CL, et al. The oceanic sink for anthropogenic CO2. Science. 2004;305:367–371. - PubMed
-
- The Royal Society. Ocean Acidification Due to Increasing Atmospheric Carbon Dioxide. 2005 Policy Document 12/05 (The Royal Society, London)
-
- Doney SC, Fabry VJ, Feely RA, Kleypas JA. Ocean acidification: The other CO2 problem. Ann Rev Mar Sci. 2009;1:169–192. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

