Nitrous oxide emission from denitrification in stream and river networks
- PMID: 21173258
- PMCID: PMC3017147
- DOI: 10.1073/pnas.1011464108
Nitrous oxide emission from denitrification in stream and river networks
Abstract
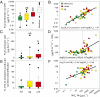
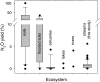
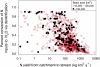
Nitrous oxide (N(2)O) is a potent greenhouse gas that contributes to climate change and stratospheric ozone destruction. Anthropogenic nitrogen (N) loading to river networks is a potentially important source of N(2)O via microbial denitrification that converts N to N(2)O and dinitrogen (N(2)). The fraction of denitrified N that escapes as N(2)O rather than N(2) (i.e., the N(2)O yield) is an important determinant of how much N(2)O is produced by river networks, but little is known about the N(2)O yield in flowing waters. Here, we present the results of whole-stream (15)N-tracer additions conducted in 72 headwater streams draining multiple land-use types across the United States. We found that stream denitrification produces N(2)O at rates that increase with stream water nitrate (NO(3)(-)) concentrations, but that <1% of denitrified N is converted to N(2)O. Unlike some previous studies, we found no relationship between the N(2)O yield and stream water NO(3)(-). We suggest that increased stream NO(3)(-) loading stimulates denitrification and concomitant N(2)O production, but does not increase the N(2)O yield. In our study, most streams were sources of N(2)O to the atmosphere and the highest emission rates were observed in streams draining urban basins. Using a global river network model, we estimate that microbial N transformations (e.g., denitrification and nitrification) convert at least 0.68 Tg·y(-1) of anthropogenic N inputs to N(2)O in river networks, equivalent to 10% of the global anthropogenic N(2)O emission rate. This estimate of stream and river N(2)O emissions is three times greater than estimated by the Intergovernmental Panel on Climate Change.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Robertson GP, Vitousek PM. Nitrogen in Agriculture: Balancing the cost of an essential resource. Annu Rev Environ Resour. 2009;34:97–125.
-
- Forster P, et al. Changes in atmospheric constituents and in radiative forcing. In: Solomon S, et al., editors. Climate Change 2007: The Physical Science Basis. Contributions of Working Group I to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change. New York: Cambridge Univ Press; 2007.
-
- Ravishankara AR, Daniel JS, Portmann RW. Nitrous oxide (N2O): The dominant ozone-depleting substance emitted in the 21st century. Science. 2009;326:123–125. - PubMed
-
- Stehfest E, Bouwman L. N2O and NO emission from agricultural fields and soils under natural vegetation: Summarizing available measurement data and modeling of global annual emissions. Nutr Cycl Agroecosyst. 2006;74:207–228.
-
- Firestone MK, Davidson EA. Microbiological basis of NO and N2O production and consumption in soil. In: Andreae MO, Schimel DS, editors. Exchange of Trace Gases Between Terrestrial Ecosystems and the Atmosphere. Chichester, UK: Wiley; 1989. pp. 7–21.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

