Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein
- PMID: 21173259
- PMCID: PMC3017144
- DOI: 10.1073/pnas.1012076108
Mechanism of RNA stabilization and translational activation by a pentatricopeptide repeat protein
Abstract
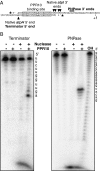
Pentatricopeptide repeat (PPR) proteins comprise a large family of helical repeat proteins that bind RNA and modulate organellar RNA metabolism. The mechanisms underlying the functions attributed to PPR proteins are unknown. We describe in vitro studies of the maize protein PPR10 that clarify how PPR10 modulates the stability and translation of specific chloroplast mRNAs. We show that recombinant PPR10 bound to its native binding site in the chloroplast atpI-atpH intergenic region (i) blocks both 5'→3' and 3'→ 5 exoribonucleases in vitro; (ii) is sufficient to define the native processed atpH mRNA 5'-terminus in conjunction with a generic 5'→3' exoribonuclease; and (iii) remodels the structure of the atpH ribosome-binding site in a manner that can account for PPR10's ability to enhance atpH translation. In addition, we show that the minimal PPR10-binding site spans 17 nt. We propose that the site-specific barrier and RNA remodeling activities of PPR10 are a consequence of its unusually long, high-affinity interface with single-stranded RNA, that this interface provides a functional mimic to bacterial small RNAs, and that analogous activities underlie many of the biological functions that have been attributed to PPR proteins.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

