Enhancement of experimental cutaneous leishmaniasis by Leishmania molecules is dependent on interleukin-4, serine protease/esterase activity, and parasite and host genetic backgrounds
- PMID: 21173308
- PMCID: PMC3067519
- DOI: 10.1128/IAI.00309-10
Enhancement of experimental cutaneous leishmaniasis by Leishmania molecules is dependent on interleukin-4, serine protease/esterase activity, and parasite and host genetic backgrounds
Abstract
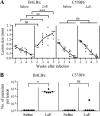
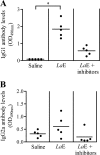
Most inbred strains of mice, like the BALB/c strain, are susceptible to Leishmania amazonensis infections and resistant to Leishmania braziliensis infections. This parasite-related difference could result from the activity of an L. amazonensis-specific virulence factor. In agreement with this hypothesis, it is shown here that the intravenous injection of BALB/c mice with L. amazonensis amastigote extract (LaE) but not the L. braziliensis extract confers susceptibility to L. braziliensis infection. This effect was associated with high circulating levels of IgG1 anti-L. amazonensis antibodies and with an increase in interleukin-4 (IL-4) production and a decrease in gamma interferon production by draining lymph node cells. Moreover, the effect was absent in IL-4-knockout mice. The biological activity in the LaE was not mediated by amphiphilic molecules and was inhibited by pretreatment of the extract with irreversible serine protease inhibitors. These findings indicate that the LaE contains a virulence-related factor that (i) enhances the Leishmania infection by promoting Th2-type immune responses, (ii) is not one of the immunomodulatory Leishmania molecules described so far, and (iii) is either a serine protease or has an effect that depends on that protease activity. In addition to being Leishmania species specific, the infection-enhancing activity was also shown to depend on the host genetic makeup, as LaE injections did not affect the susceptibility of C57BL/6 mice to L. braziliensis infection. The identification of Leishmania molecules with infection-enhancing activity could be important for the development of a vaccine, since the up- or downmodulation of the immune response against a virulence factor could well contribute to controlling the infection.
Figures




Similar articles
-
Enhancement of experimental cutaneous leishmaniasis by Leishmania extract: identification of a disease-associated antibody specificity.BMC Res Notes. 2015 May 14;8:197. doi: 10.1186/s13104-015-1158-0. BMC Res Notes. 2015. PMID: 25971623 Free PMC article.
-
Leishmania braziliensis and Leishmania amazonensis amastigote extracts differ in their enhancement effect on Leishmania infection when injected intradermally.BMC Res Notes. 2014 Feb 1;7:70. doi: 10.1186/1756-0500-7-70. BMC Res Notes. 2014. PMID: 24484604 Free PMC article.
-
Antigenic extracts of Leishmania braziliensis and Leishmania amazonensis associated with saponin partially protects BALB/c mice against Leishmania chagasi infection by suppressing IL-10 and IL-4 production.Mem Inst Oswaldo Cruz. 2010 Sep;105(6):818-22. doi: 10.1590/s0074-02762010000600015. Mem Inst Oswaldo Cruz. 2010. PMID: 20944999
-
Immunopathogenic competences of Leishmania (V.) braziliensis and L. (L.) amazonensis in American cutaneous leishmaniasis.Parasite Immunol. 2009 Aug;31(8):423-31. doi: 10.1111/j.1365-3024.2009.01116.x. Parasite Immunol. 2009. PMID: 19646206 Review.
-
The Paradox of a Phagosomal Lifestyle: How Innate Host Cell-Leishmania amazonensis Interactions Lead to a Progressive Chronic Disease.Front Immunol. 2021 Sep 7;12:728848. doi: 10.3389/fimmu.2021.728848. eCollection 2021. Front Immunol. 2021. PMID: 34557194 Free PMC article. Review.
Cited by
-
Enhancement of experimental cutaneous leishmaniasis by Leishmania extract: identification of a disease-associated antibody specificity.BMC Res Notes. 2015 May 14;8:197. doi: 10.1186/s13104-015-1158-0. BMC Res Notes. 2015. PMID: 25971623 Free PMC article.
-
Leishmania braziliensis and Leishmania amazonensis amastigote extracts differ in their enhancement effect on Leishmania infection when injected intradermally.BMC Res Notes. 2014 Feb 1;7:70. doi: 10.1186/1756-0500-7-70. BMC Res Notes. 2014. PMID: 24484604 Free PMC article.
-
Leishmania (Viannia) shawi purified antigens confer protection against murine cutaneous leishmaniasis.Inflamm Res. 2012 Mar;61(3):255-63. doi: 10.1007/s00011-011-0407-5. Epub 2011 Dec 14. Inflamm Res. 2012. PMID: 22166919
-
Mechanisms of immune evasion in leishmaniasis.Adv Appl Microbiol. 2013;82:155-84. doi: 10.1016/B978-0-12-407679-2.00005-3. Adv Appl Microbiol. 2013. PMID: 23415155 Free PMC article.
References
-
- Aebischer, T., L. Morris, and E. Handman. 1994. Intravenous injection of irradiated Leishmania major into susceptible BALB/c mice: immunization or protective tolerance. Int. Immunol. 6:1535-1543. - PubMed
-
- Aida, Y., and M. J. Pabst. 1990. Removal of endotoxin from protein solutions by phase separation using Triton X-114. J. Immunol. Methods 132:191-195. - PubMed
-
- Ashford, R. W., P. Desjeux, and P. Deraadt. 1999. Estimation of population at risk of infection and number of cases of leishmaniasis. Parasitol. Today 8:104-105. - PubMed
-
- Buxbaum, L. U., et al. 2003. Cysteine protease B of Leishmania mexicana inhibits host Th1 responses and protective immunity. J. Immunol. 171:3711-3717. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

