Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene
- PMID: 21173351
- PMCID: PMC3061281
- DOI: 10.1161/CIRCULATIONAHA.110.970673
Mitogen-activated protein kinase inhibitors improve heart function and prevent fibrosis in cardiomyopathy caused by mutation in lamin A/C gene
Abstract
Background: Mutations in the lamin A/C gene, LMNA, can cause dilated cardiomyopathy. We have shown abnormal activation of the extracellular signal-regulated kinase (ERK) and the c-jun N-terminal kinase (JNK) branches of the mitogen-activated protein kinase signaling cascade in hearts from Lmna(H222P/H222P) mice that develop dilated cardiomyopathy. We recently showed that partial inhibition of ERK and JNK signaling before the onset of cardiomyopathy in Lmna(H222P/H222P) mice prevented the development of left ventricle dilatation and decreased cardiac ejection fraction at a time when they occurred in untreated mice.
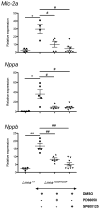
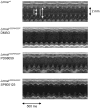
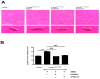
Methods and results: To determine whether pharmacological inhibitors of ERK and JNK signaling could be clinically useful to treat cardiomyopathy caused by LMNA mutation, we administered them to Lmna(H222P/H222P) mice after they developed left ventricular dilatation and decreased ejection fraction. Lmna(H222P/H222P) mice were treated with ERK and JNK signaling inhibitors from 16 to 20 or, in pilot experiments, 19 to 24 weeks of age. The inhibitors blocked increased expression of RNAs encoding natriuretic peptide precursors and proteins involved in sarcomere architecture that occurred in placebo-treated mice. Echocardiography and histological analysis demonstrated that treatment prevented left ventricular end-systolic dilatation, increased ejection fraction, and decreased myocardial fibrosis.
Conclusion: Inhibitors of ERK and JNK signaling could potentially be used to treat humans with cardiomyopathy caused by LMNA mutations.
Figures



References
-
- Bonne G, Di Barletta MR, Varnous S, Becane HM, Hammouda EH, Merlini L, Muntoni F, Greenberg CR, Gary F, Urtizberea JA, Duboc D, Fardeau M, Toniolo D, Schwartz K. Mutations in the gene encoding lamin A/C cause autosomal dominant Emery-Dreifuss muscular dystrophy. Nat Genet. 1999;21:285–288. - PubMed
-
- Muchir A, Bonne G, van der Kooi AJ, van Meegen M, Baas F, Bolhuis PA, de Visser M, Schwartz K. Identification of mutations in the gene encoding lamins A/C in autosomal dominant limb girdle muscular dystrophy with atrioventricular conduction disturbances (LGMD1B) Hum Mol Genet. 2000;9:1453–1459. - PubMed
-
- Fatkin D, MacRae C, Sasaki T, Wolff MR, Porcu M, Frenneaux M, Atherton J, Vidaillet HJ, Jr, Spudich S, De Girolami U, Seidman JG, Seidman C, Muntoni F, Müehle G, Johnson W, McDonough B. Missense mutations in the rod domain of the lamin A/C gene as causes of dilated cardiomyopathy and conduction-system disease. N Engl J Med. 1999;341:1715–1724. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

