Non-clustered protocadherin
- PMID: 21173574
- PMCID: PMC3084973
- DOI: 10.4161/cam.5.2.14374
Non-clustered protocadherin
Abstract
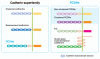
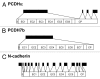
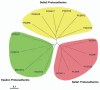
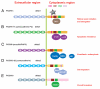
The cadherin family is classified into classical cadherins, desmosomal cadherins and protocadherins (PCDHs). Genomic structures distinguish between PCDHs and other cadherins, and between clustered and non-clustered PCDHs. The phylogenetic analysis with full sequences of non-clustered PCDHs enabled them to be further classified into three subgroups: δ1 (PCDH1, PCDH7, PCDH9, PCDH11 and PCDH20), δ2 (PCDH8, PCDH10, PCDH12, PCDH17, PCDH18 and PCDH19) and ε (PCDH15, PCDH16, PCDH21 and MUCDHL). ε-PCDH members except PCDH21 have either higher or lower numbers of cadherin repeats than those of other PCDHs. Non-clustered PCDHs are expressed predominantly in the nervous system and have spatiotemporally diverse expression patterns. Especially, the region-specific expressions of non-clustered PCDHs have been observed in cortical area of early postnatal stage and in caudate putaman and/or hippocampal formation of mature brains, suggesting that non-clustered PCDHs play roles in the circuit formation and maintenance. The non-clustered PCDHs appear to have homophilic/heterophilc cell-cell adhesion properties, and each member has diverse cell signaling partnership distinct from those of other members (PCDH7/TAF1; PCDH8/TAO2β; PCDH10/Nap1; PCDH11/β-catenin; PCDH18/mDab1). Furthermore, each PCDH has several isoforms with differential cytoplasmic sequences, suggesting that one PCDH isoform could activate intracellular signaling differential from other isoforms. These facts suggest that non-clustered PCDHs play roles as a mediator of a regulator of other molecules as well as cell-cell adhesion. Furthermore, some non-clustered PCDHs have been considered to be involved in neuronal diseases such as autism-spectrum disorders, schizophrenia, and female-limited epilepsy and cognitive impairment, suggesting that they play multiple, tightly regulated roles in normal brain function. In addition, some non-clustered PCDHs have been suggested as candidate tumor suppressor genes in several tissues. Although molecular adhesive and regulatory properties of some PCDHs began to be unveiled, the endeavor to understand the molecular mechanism of non-clustered PCDH is still in its infancy and requires future study.
Figures

References
-
- Nollet F, Kools P, van Roy F. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. - PubMed
-
- Halbleib JM, Nelson WJ. Cadherins in development: cell adhesion, sorting and tissue morphogenesis. Genes Dev. 2006;20:3199–3214. - PubMed
-
- Morishita H, Yagi T. Protocadherin family: diversity, structure and function. Curr Opin Cell Biol. 2007;19:584–592. - PubMed
-
- Wu Q, Maniatis T. A striking organization of a large family of human neural cadherin-like cell adhesion genes. Cell. 1999;97:779–790. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
