H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses
- PMID: 21174144
- PMCID: PMC3132412
- DOI: 10.1007/s10875-010-9490-6
H5N1 influenza vaccine formulated with AS03 A induces strong cross-reactive and polyfunctional CD4 T-cell responses
Abstract
Objective: Adjuvantation of an H5N1 split-virion influenza vaccine with AS03(A) substantially reduces the antigen dose required to produce a putatively protective humoral response and promotes cross-clade neutralizing responses. We determined the effect of adjuvantation on antibody persistence and B- and T-cell-mediated immune responses.
Methods: Two vaccinations with a split-virion A/Vietnam/1194/2004 (H5N1, clade 1) vaccine containing 3.75-30 μg hemagglutinin and formulated with or without adjuvant were administered to groups of 50 volunteers aged 18-60 years.
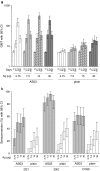
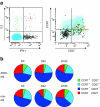
Results: Adjuvantation of the vaccine led to better persistence of neutralizing and hemagglutination-inhibiting antibodies and higher frequencies of antigen-specific memory B cells. Cross-reactive and polyfunctional H5N1-specific CD4 T cells were detected at baseline and were amplified by vaccination. Expansion of CD4 T cells was enhanced by adjuvantation.
Conclusion: Formulation of the H5N1 vaccine with AS03(A) enhances antibody persistence and induces stronger T- and B-cell responses. The cross-clade T-cell immunity indicates that the adjuvanted vaccine primes individuals to respond to either infection and/or subsequent vaccination with strains drifted from the primary vaccine strain.
Figures






References
-
- World Health Organization. Avian influenza. World Health Organization. 2009. http://www.who.int/csr/disease/avian_influenza/en/index.html. Accessed 27 Aug 2010.
-
- European Agency for Proprietary Medicinal Products. Guideline on influenza vaccine prepared from viruses with the potential to cause a pandemic and intended for use outside of the core dossier context (EMEA/CHMP/VWP/263499/2006). European Agency for the Evaluation of Medicinal Products; 2007.
-
- Food and Drug Administration. Guidance for industry: clinical data needed to support the licensure of pandemic influenza vaccines. 2007.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

