Unique dynamic properties of DNA duplexes containing interstrand cross-links
- PMID: 21174443
- PMCID: PMC3032004
- DOI: 10.1021/bi101813h
Unique dynamic properties of DNA duplexes containing interstrand cross-links
Abstract
Bifunctional DNA alkylating agents form a diverse assortment of covalent DNA interstrand cross-linked (ICL) structures that are potent cytotoxins. Because it is implausible that cells could possess distinct DNA repair systems for each individual ICL, it is believed that common structural and dynamic features of ICL damage are recognized, rather than specific structural characteristics of each cross-linking agent. Investigation of the structural and dynamic properties of ICLs that might be important for recognition has been complicated by heterogeneous incorporation of these lesions into DNA. To address this problem, we have synthesized and characterized several homogeneous ICL DNAs containing site-specific staggered N4-cytosine-ethyl-N4-cytosine cross-links. Staggered cross-links were introduced in two ways, in a manner that preserves the overall structure of B-form duplex DNA and in a manner that highly distorts the DNA structure, with the goal of understanding how structural and dynamic properties of diverse ICL duplexes might flag these sites for repair. Measurements of base pair opening dynamics in the B-form ICL duplex by (1)H NMR line width or imino proton solvent exchange showed that the guanine base opposite the cross-linked cytosine opened at least 1 order of magnitude more slowly than when in a control matched normal duplex. To a lesser degree, the B-form ICL also induced a decrease in base pair opening dynamics that extended from the site of the cross-link to adjacent base pairs. In contrast, the non-B-form ICL showed extensive conformational dynamics at the site of the cross-link, which extended over the entire DNA sequence. Because DNA duplexes containing the B-form and non-B-form ICL cross-links have both been shown to be incised when incubated in mammalian whole cell extracts, while a matched normal duplex is not, we conclude that intrinsic DNA dynamics is not a requirement for specific damage incision of these ICLs. Instead, we propose a general model in which destabilized ICL duplexes serve to energetically facilitate binding of DNA repair factors that must induce bubbles or other distortions in the duplex. However, the essential requirement for incision is an immobile Y-junction where the repair factors are stably bound at the site of the ICL, and the two DNA strands are unpaired.
Figures

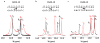
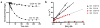

Similar articles
-
N(4)C-ethyl-N(4)C cross-linked DNA: synthesis and characterization of duplexes with interstrand cross-links of different orientations.Biochemistry. 2002 Jan 22;41(3):760-71. doi: 10.1021/bi011610u. Biochemistry. 2002. PMID: 11790097
-
Mispair-aligned N3T-alkyl-N3T interstrand cross-linked DNA: synthesis and characterization of duplexes with interstrand cross-links of variable lengths.J Am Chem Soc. 2004 Aug 4;126(30):9257-65. doi: 10.1021/ja0498540. J Am Chem Soc. 2004. PMID: 15281815
-
Electrochemical properties of interstrand cross-linked DNA duplexes labeled with Nile blue.Langmuir. 2012 Dec 11;28(49):17211-6. doi: 10.1021/la3036538. Epub 2012 Nov 26. Langmuir. 2012. PMID: 23153070
-
Advances in understanding the complex mechanisms of DNA interstrand cross-link repair.Cold Spring Harb Perspect Biol. 2013 Oct 1;5(10):a012732. doi: 10.1101/cshperspect.a012732. Cold Spring Harb Perspect Biol. 2013. PMID: 24086043 Free PMC article. Review.
-
The Induction and Repair of DNA Interstrand Crosslinks and Implications in Cancer Chemotherapy.Anticancer Agents Med Chem. 2015;16(2):221-46. doi: 10.2174/1871520615666150824160421. Anticancer Agents Med Chem. 2015. PMID: 26299659 Review.
Cited by
-
Base-pair Opening Dynamics of Nucleic Acids in Relation to Their Biological Function.Comput Struct Biotechnol J. 2019 Jun 13;17:797-804. doi: 10.1016/j.csbj.2019.06.008. eCollection 2019. Comput Struct Biotechnol J. 2019. PMID: 31312417 Free PMC article. Review.
-
NMR analysis of base-pair opening kinetics in DNA.Curr Protoc Nucleic Acid Chem. 2014 Dec 12;59:7.20.1-18. doi: 10.1002/0471142700.nc0720s59. Curr Protoc Nucleic Acid Chem. 2014. PMID: 25501592 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

