Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury
- PMID: 21175261
- PMCID: PMC5206695
- DOI: 10.1089/neu.2010.1648
Fluoxetine increases hippocampal neurogenesis and induces epigenetic factors but does not improve functional recovery after traumatic brain injury
Abstract
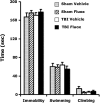
The selective serotonin reuptake inhibitor fluoxetine induces hippocampal neurogenesis, stimulates maturation and synaptic plasticity of adult hippocampal neurons, and reduces motor/sensory and memory impairments in several CNS disorders. In the setting of traumatic brain injury (TBI), its effects on neuroplasticity and function have yet to be thoroughly investigated. Here we examined the efficacy of fluoxetine after a moderate to severe TBI, produced by a controlled cortical impact. Three days after TBI or sham surgery, mice were treated with fluoxetine (10 mg/kg/d) or vehicle for 4 weeks. To evaluate the effects of fluoxetine on neuroplasticity, hippocampal neurogenesis and epigenetic modification were studied. Stereologic analysis of the dentate gyrus revealed a significant increase in doublecortin-positive cells in brain-injured animals treated with fluoxetine relative to controls, a finding consistent with enhanced hippocampal neurogenesis. Epigenetic modifications, including an increase in histone 3 acetylation and induction of methyl-CpG-binding protein, a transcription factor involved in DNA methylation, were likewise seen by immunohistochemistry and quantitative Western immunoblots, respectively, in brain-injured animals treated with fluoxetine. To determine if fluoxetine improves neurological outcomes after TBI, gait function and spatial learning and memory were assessed by the CatWalk-assisted gait test and Barnes maze test, respectively. No differences in these parameters were seen between fluoxetine- and vehicle-treated animals. Thus while fluoxetine enhanced neuroplasticity in the hippocampus after TBI, its chronic administration did not restore locomotor function or ameliorate memory deficits.
Conflict of interest statement
Author Disclosure Statement No competing financial interests exist.
Figures





References
-
- Beauquis J. Roig P. De Nicola A.F. Saravia F. Neuronal plasticity and antidepressants in the diabetic brain. Ann. NY Acad. Sci. 2009;1153:203–208. - PubMed
-
- Berends H.I. Nijlant J. van Putten M. Movig K.L. Single dose of fluoxetine increases muscle activation in chronic stroke patients. Clin. Neuropharmacol. 2009;32:1–5. - PubMed
-
- Bilgen M. A new device for experimental modeling of central nervous system injuries. Neurorehabil. Neural Repair. 2005;19:219–226. - PubMed
-
- Boast C. Bartolomeo A.C. Morris H. Moyer J.A. 5HT antagonists attenuate MK801-impaired radial arm maze performance in rats. Neurobiol. Learn. Mem. 1999;71:259–271. - PubMed
-
- Boyeson M.G. Harmon R.L. Jones J.L. Comparative effects of fluoxetine, amitriptyline and serotonin on functional motor recovery after sensorimotor cortex injury. Am. J. Phys. Med. Rehabil. 1994;73:76–83. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

