Microwave tissue ablation: biophysics, technology, and applications
- PMID: 21175404
- PMCID: PMC3058696
- DOI: 10.1615/critrevbiomedeng.v38.i1.60
Microwave tissue ablation: biophysics, technology, and applications
Abstract
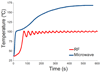
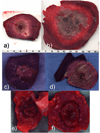
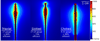
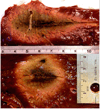
Microwave ablation is an emerging treatment option for many cancers, cardiac arrhythmias, and other medical conditions. During treatment, microwaves are applied directly to tissues to produce rapid temperature elevations sufficient to produce immediate coagulative necrosis. The engineering design criteria for each application differ, with individual consideration for factors such as desired ablation zone size, treatment duration, and procedural invasiveness. Recent technological developments in applicator cooling, power control, and system optimization for specific applications promise to increase the utilization of microwave ablation in the future. This article reviews the basic biophysics of microwave tissue heating, provides an overview of the design and operation of current equipment, and outlines areas for future research.
Figures





References
-
- Bakker JF, Paulides MM, Westra AH, Schippers H, Van Rhoon GC. Design and test of a 434 MHz multi-channel amplifier system for targeted hyperthermia applicators. Int J Hyperthermia. 2010;26:158–170. - PubMed
-
- Converse M, Bond EJ, Van Veen BD, Hagness SC. A computational study of ultra-wideband versus narrowband microwave hyperthermia for breast cancer treatment. IEEE Trans Microw Theory Tech. 2006;54:2169–2180.
-
- Balanis CA. Advanced engineering electromagnetics. New York: John Wiley & Sons; 1989.
-
- Pennes HH. Temperature of skeletal muscle in cerebral hemiplegia and paralysis agitans. Arch Neurol Psychiatry. 1949;62:269–279. - PubMed
-
- Gabriel S, Lau RW, Gabriel C. The dielectric properties of biological tissues: ii. measurements in the frequency range 10 hz to 20 GHz. Phys Med Biol. 1996;41:2251–2269. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

