Randomized trial evaluating serial protein C levels in severe sepsis patients treated with variable doses of drotrecogin alfa (activated)
- PMID: 21176144
- PMCID: PMC3219981
- DOI: 10.1186/cc9382
Randomized trial evaluating serial protein C levels in severe sepsis patients treated with variable doses of drotrecogin alfa (activated)
Abstract
Introduction: Serial alterations in protein C levels appear to correlate with disease severity in patients with severe sepsis, and it may be possible to tailor severe sepsis therapy with the use of this biomarker. The purpose of this study was to evaluate the dose and duration of drotrecogin alfa (activated) treatment using serial measurements of protein C compared to standard therapy in patients with severe sepsis.
Methods: This was a phase 2 multicenter, randomized, double-blind, controlled study. Adult patients with two or more sepsis-induced organ dysfunctions were enrolled. Protein C deficient patients were randomized to standard therapy (24 μg/kg/hr infusion for 96 hours) or alternative therapy (higher dose and/or variable duration; 24/30/36 μg/kg/hr for 48 to 168 hours). The primary outcome was a change in protein C level in the alternative therapy group, between study Day 1 and Day 7, compared to standard therapy.
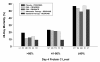
Results: Of 557 patients enrolled, 433 patients received randomized therapy; 206 alternative, and 227 standard. Baseline characteristics of the groups were largely similar. The difference in absolute change in protein C from Day 1 to Day 7 between the two therapy groups was 7% (P = 0.011). Higher doses and longer infusions were associated with a more pronounced increase in protein C level, with no serious bleeding events. The same doses and longer infusions were associated with a larger increase in protein C level; higher rates of serious bleeding when groups received the same treatment; but no clear increased risk of bleeding during the longer infusion. This group also experienced a higher mortality rate; however, there was no clear link to infusion duration.
Conclusions: The study met its primary objective of increased protein C levels in patients receiving alternative therapy demonstrating that variable doses and/or duration of drotrecogin alfa (activated) can improve protein C levels, and also provides valuable information for incorporation into potential future studies.
Trial registration: ClinicalTrials.gov identifier: NCT00386425.
Figures



Comment in
-
Treatment with recombinant human activated protein C: one size does not fit all.Crit Care. 2011 Jan 17;15(1):105. doi: 10.1186/cc9375. Crit Care. 2011. PMID: 21247509 Free PMC article.
References
-
- Levy MM, Dellinger RP, Townsend SR, Linde-Zwirble WT, Marshall JC, Bion J, Schorr C, Artigas A, Ramsay G, Beale R, Parker MM, Gerlach H, Reinhart K, Silva E, Harvey M, Regan S, Angus DC. Surviving Sepsis Campaign. The Surviving Sepsis Campaign: results of an international guideline-based performance improvement program targeting severe sepsis. Crit Care Med. 2010;38:367–374. doi: 10.1097/CCM.0b013e3181cb0cdc. - DOI - PubMed
-
- Bernard GR, Vincent JL, Laterre PF, LaRosa SP, Dhainaut JF, Lopez-Rodriguez A, Steingrub JS, Garber GE, Helterbrand JD, Ely EW, Fisher CJ Jr. Recombinant human protein C Worldwide Evaluation in Severe Sepsis (PROWESS) study group. Efficacy and safety of recombinant activated protein C for severe sepsis. N Engl J Med. 2001;344:699–709. doi: 10.1056/NEJM200103083441001. - DOI - PubMed
-
- Kinasewitz GT, Yan SB, Basson B, Comp P, Russell JA, Cariou A, Um SL, Utterback B, Laterre P-F, Dhainaut J-F. for the PROWESS Sepsis Study Group. Universal changes in biomarkers of coagulation and inflammation occur in patients with severe sepsis, regardless of causative micro-organism. Crit Care. 2004;8:R82–R90. doi: 10.1186/cc2459. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

