A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse
- PMID: 21176145
- PMCID: PMC3017038
- DOI: 10.1186/1471-213X-10-121
A sensitive and bright single-cell resolution live imaging reporter of Wnt/ß-catenin signaling in the mouse
Abstract
Background: Understanding the dynamic cellular behaviors and underlying molecular mechanisms that drive morphogenesis is an ongoing challenge in biology. Live imaging provides the necessary methodology to unravel the synergistic and stereotypical cell and molecular events that shape the embryo. Genetically-encoded reporters represent an essential tool for live imaging. Reporter strains can be engineered by placing cis-regulatory elements of interest to direct the expression of a desired reporter gene. In the case of canonical Wnt signaling, also referred to as Wnt/β-catenin signaling, since the downstream transcriptional response is well understood, reporters can be designed that reflect sites of active Wnt signaling, as opposed to sites of gene transcription, as is the case with many fluorescent reporters. However, even though several transgenic Wnt/β-catenin reporter strains have been generated, to date, none provides the single-cell resolution favored for live imaging studies.
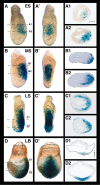
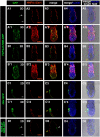
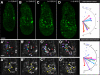
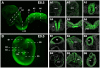
Results: We have placed six copies of a TCF/Lef responsive element and an hsp68 minimal promoter in front of a fluorescent protein fusion comprising human histone H2B to GFP and used it to generate a strain of mice that would report Wnt/β-catenin signaling activity. Characterization of developmental and adult stages of the resulting TCF/Lef:H2B-GFP strain revealed discrete and specific expression of the transgene at previously characterized sites of Wnt/β-catenin signaling. In support of the increased sensitivity of the TCF/Lef:H2B-GFP reporter, additional sites of Wnt/β-catenin signaling not documented with other reporters but identified through genetic and embryological analysis were observed. Furthermore, the sub-cellular localization of the reporter minimized reporter perdurance, and allowed visualization and tracking of individual cells within a cohort, so facilitating the detailed analysis of cell behaviors and signaling activity during morphogenesis.
Conclusion: By combining the Wnt activity read-out efficiency of multimerized TCF/Lef DNA binding sites, together with the high-resolution imaging afforded by subcellularly-localized fluorescent fusion proteins such as H2B-GFP, we have created a mouse transgenic line that faithfully recapitulates Wnt signaling activity at single-cell resolution. The TCF/Lef:H2B-GFP reporter represents a unique tool for live imaging the in vivo processes triggered by Wnt/β-catenin signaling, and thus should help the formulation of a high-resolution understanding of the serial events that define the morphogenetic process regulated by this signaling pathway.
Figures






References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

