Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection
- PMID: 21176204
- PMCID: PMC3022776
- DOI: 10.1186/1741-7007-8-152
Drosophila EGFR pathway coordinates stem cell proliferation and gut remodeling following infection
Abstract
Background: Gut homeostasis is central to whole organism health, and its disruption is associated with a broad range of pathologies. Following damage, complex physiological events are required in the gut to maintain proper homeostasis. Previously, we demonstrated that ingestion of a nonlethal pathogen, Erwinia carotovora carotovora 15, induces a massive increase in stem cell proliferation in the gut of Drosophila. However, the precise cellular events that occur following infection have not been quantitatively described, nor do we understand the interaction between multiple pathways that have been implicated in epithelium renewal.
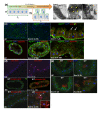
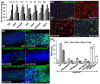
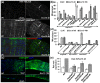
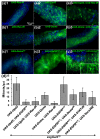
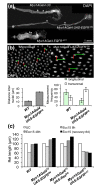
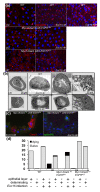
Results: To understand the process of infection and epithelium renewal in more detail, we performed a quantitative analysis of several cellular and morphological characteristics of the gut. We observed that the gut of adult Drosophila undergoes a dynamic remodeling in response to bacterial infection. This remodeling coordinates the synthesis of new enterocytes, their proper morphogenesis and the elimination of damaged cells through delamination and anoikis. We demonstrate that one signaling pathway, the epidermal growth factor receptor (EGFR) pathway, is key to controlling each of these steps through distinct functions in intestinal stem cells and enterocytes. The EGFR pathway is activated by the EGF ligands, Spitz, Keren and Vein, the latter being induced in the surrounding visceral muscles in part under the control of the Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathway. Additionally, the EGFR pathway synergizes with the JAK/STAT pathway in stem cells to promote their proliferation. Finally, we show that the EGFR pathway contributes to gut morphogenesis through its activity in enterocytes and is required to properly coordinate the delamination and anoikis of damaged cells. This function of the EGFR pathway in enterocytes is key to maintaining homeostasis, as flies lacking EGFR are highly susceptible to infection.
Conclusions: This study demonstrates that restoration of normal gut morphology following bacterial infection is a more complex phenomenon than previously described. Maintenance of gut homeostasis requires the coordination of stem cell proliferation and differentiation, with the incorporation and morphogenesis of new cells and the expulsion of damaged enterocytes. We show that one signaling pathway, the EGFR pathway, is central to all these stages, and its activation at multiple steps could synchronize the complex cellular events leading to gut repair and homeostasis.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

