Modulation of HIV-1-host interaction: role of the Vpu accessory protein
- PMID: 21176220
- PMCID: PMC3022690
- DOI: 10.1186/1742-4690-7-114
Modulation of HIV-1-host interaction: role of the Vpu accessory protein
Abstract
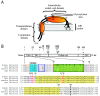
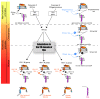
Viral protein U (Vpu) is a type 1 membrane-associated accessory protein that is unique to human immunodeficiency virus type 1 (HIV-1) and a subset of related simian immunodeficiency virus (SIV). The Vpu protein encoded by HIV-1 is associated with two primary functions during the viral life cycle. First, it contributes to HIV-1-induced CD4 receptor downregulation by mediating the proteasomal degradation of newly synthesized CD4 molecules in the endoplasmic reticulum (ER). Second, it enhances the release of progeny virions from infected cells by antagonizing Tetherin, an interferon (IFN)-regulated host restriction factor that directly cross-links virions on host cell-surface. This review will mostly focus on recent advances on the role of Vpu in CD4 downregulation and Tetherin antagonism and will discuss how these two functions may have impacted primate immunodeficiency virus cross-species transmission and the emergence of pandemic strain of HIV-1.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

