High-throughput cryopreservation of spermatozoa of blue catfish (Ictalurus furcatus): Establishment of an approach for commercial-scale processing
- PMID: 21176772
- PMCID: PMC3509363
- DOI: 10.1016/j.cryobiol.2010.12.006
High-throughput cryopreservation of spermatozoa of blue catfish (Ictalurus furcatus): Establishment of an approach for commercial-scale processing
Abstract
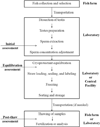
Hybrid catfish created by crossing of female channel catfish (Ictalurus punctatus) and male blue catfish (Ictalurus furcatus) are being used increasingly in foodfish aquaculture because of their fast growth and efficient food conversion. However, the availability of blue catfish males is limited, and their peak spawning is at a different time than that of the channel catfish. As such, cryopreservation of sperm of blue catfish could improve production of hybrid catfish, and has been studied in the laboratory and tested for feasibility in a commercial dairy bull cryopreservation facility. However, an approach for commercially relevant production of cryopreserved blue catfish sperm is still needed. The goal of this study was to develop practical approaches for commercial-scale sperm cryopreservation of blue catfish by use of an automated high-throughput system (MAPI, CryoBioSystem Co.). The objectives were to: (1) refine cooling rate and cryoprotectant concentration, and evaluate their interactions; (2) evaluate the effect of sperm concentration on cryopreservation; (3) refine cryoprotectant concentration based on the highest effective sperm concentration; (4) compare the effect of thawing samples at 20 or 40°C; (5) evaluate the fertility of thawed sperm at a research scale by fertilizing with channel catfish eggs; (6) test the post-thaw motility and fertility of sperm from individual males in a commercial setting, and (7) test for correlation of cryopreservation results with biological indices used for male evaluation. The optimal cooling rate was 5°C/min (Micro Digitcool, IMV) for high-throughput cryopreservation using CBS high-biosecurity 0.5-ml straws with 10% methanol, and a concentration of 1×10(9)sperm/ml. There was no difference in post-thaw motility when samples were thawed at 20°C for 40s or 40°C for 20s. After fertilization, the percentage of neurulation (Stage V embryos) was 80±21%, and percentage of embryonic mobility (Stage VI embryo) was 51±22%. There was a significant difference among the neurulation values produced by thawed blue catfish sperm, fresh blue catfish sperm (P=0.010) and channel catfish sperm (P=0.023), but not for Stage VI embryos (P≥0.585). Cryopreserved sperm from ten males did not show significant variation in post-thaw motility or fertility at the neurulation stage. This study demonstrates that the protocol established for high-throughput cryopreservation of blue catfish sperm can provide commercially relevant quantities and quality of sperm with stable fertility for hybrid catfish production and provides a model for establishment of commercial-scale approaches for other aquatic species.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures
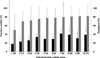
References
-
- Avery J, Steeby J, Bosworth BG, Small BC. In: Producing hybrid catfish fry: workshop manual. USDA-ARS Catfish Genetics Research Unit; Mississippi State University National Warmwater Aquaculture Center, editor. Stoneville, MS: Mississippi State University, Delta Branch Experiment Station; 2005.
-
- Bart AN, Dunham RA. Effects of sperm concentration and egg number on fertilization efficiency with channel catfish (Ictalurus punctatus) eggs and blue catfish (Ictalurus furcatus) spermatozoa. Theriogenology. 1996;45:673–682. - PubMed
-
- Bart AN, Wolfe DF, Dunham RA. Cryopreservation of blue catfish spermatozoa and subsequent fertilization of channel catfish eggs. Transactions of the American Fisheries Society. 1998;127:819–824.
-
- Bates MC, Tiersch TR. Preliminary studies of artificial spawning of channel catfish as male-females pairs or all-famale groups in recirculating systems. Journal of the World Aquaculture Society. 1998;29:325–334.
-
- Bates MC, Wayman WR, Tiersch TR. Effect of osmotic pressure on the activation and storage of channel catfish sperm. Transactions of the American Fisheries Society. 1996;125:798–802.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

