The safety of modafinil in combination with oral ∆9-tetrahydrocannabinol in humans
- PMID: 21176784
- PMCID: PMC3645879
- DOI: 10.1016/j.pbb.2010.12.013
The safety of modafinil in combination with oral ∆9-tetrahydrocannabinol in humans
Abstract
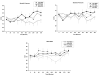
Marijuana (cannabis) is the most widely used illicit substance globally, and cannabis use is associated with a range of adverse consequences. Currently, no medications have been proven to be effective for the treatment of cannabis addiction. The goals of this study were to examine the safety and efficacy of a potential treatment medication, modafinil, in combination with oral ∆9-tetrahydrocannabinol (THC). Twelve male and female occasional cannabis users participated in an outpatient double-blind, placebo-controlled, crossover study. Across four sessions, participants were randomly assigned to a sequence of four oral treatments: (1) 400 mg modafinil+placebo, (2) 15 mg THC+placebo, (3) 400 mg modafinil+15 mg THC, or (4) placebo+placebo. Outcome measures included heart rate, blood pressure, performance on the Rapid Visual Information Processing (RVIP), and the Hopkins Verbal Learning Test (HVLT), and subjective measures. Oral THC increased heart rate, and produced increased subjective ratings of feeling "high" and "sedated," as well as increased ratings of euphoria. Modafinil alone increased the Profiles of Mood States (POMS) subscales of vigor and tension. These findings support the safety of modafinil in combination with THC. The effects of modafinil in combination with a range of doses of THC need to be determined in future studies.
Published by Elsevier Inc.
Figures


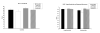
References
-
- Ballon JS, Feifel D. A systematic review of modafinil: Potential clinical uses and mechanisms of action. J Clin Psychiatry. 2006;67:554–66. - PubMed
-
- Benedict R, Schretlen D, Groninger L, Brandt J. Hopkins Verbal Learning Test-Revised: Normative Data and Analysis of Inter-Form and Test-Retest Reliability. The Clinical Neuropsychologist. 1998;12:43–55.
-
- Bolla KI, Brown K, Eldreth D, Tate K, Cadet JL. Dose-related neurocognitive effects of marijuana use. Neurology. 2002;59:1337–43. - PubMed
-
- Budney AJ, Hughes JR. The cannabis withdrawal syndrome. Curr Opin Psychiatry. 2006;19:233–8. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

