Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice
- PMID: 21176785
- PMCID: PMC3096756
- DOI: 10.1016/j.bbi.2010.12.008
Neuroinflammation modulates distinct regional and temporal clinical responses in ALS mice
Abstract
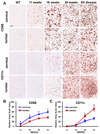
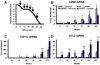
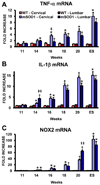
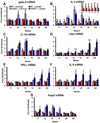
An inflammatory response is a pathological hallmark of amyotrophic lateral sclerosis (ALS), a relentless and devastating degenerative disease of motoneurons. This response is not simply a late consequence of motoneuron degeneration, but actively contributes to the balance between neuroprotection and neurotoxicity; initially infiltrating lymphocytes and microglia slow disease progression, while later, they contribute to the acceleration of disease. Since motor weakness begins in the hindlimbs of ALS mice and only later involves the forelimbs, we determined whether differential protective versus injurious inflammatory responses in the cervical and lumbar spinal cords explained the temporally distinct clinical disease courses between the limbs of these mice. Densitometric evaluation of immunohistochemical sections and quantitative RT-PCR (qRT-PCR) demonstrated that CD68 and CD11c were differentially increased in their spinals cords. qRT-PCR revealed that protective and anti-inflammatory factors, including BDNF, GDNF, and IL-4, were increased in the cervical region compared with the lumbar region. In contrast, the toxic markers TNF-α, IL-1β and NOX2 were not different between ALS mice cervical and lumbar regions. T lymphocytes were observed infiltrating lumbar spinal cords of ALS mice prior to the cervical region; mRNA levels of the transcription factor gata-3 (Th2 response) were differentially elevated in the cervical cord of ALS mice whereas t-bet (Th1 response) was increased in the lumbar cord. These results reinforce the important balance between specific protective/injurious inflammatory immune responses in modulating clinical outcomes and suggest that the delayed forelimb motor weakness in ALS mice is partially explained by augmented protective responses in the cervical spinal cords.
Copyright © 2010 Elsevier Inc. All rights reserved.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures

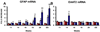

References
-
- Ajami B, Bennett JL, Krieger C, Tetzlaff W, Rossi FMV. Local self-renewal can sustain CNS microglia maintenance and function throughout adult life. Nat. Neurosci. 2007;10:1538–1543. - PubMed
-
- Arvin KL, Han BH, Du Y, Lin SZ, Paul SM, Holtzman DM. Minocycline markedly protects the neonatal brain against hypoxic-ischemic injury. Ann Neurol. 2002;52:54–61. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

