The HTLV-1 hbz antisense gene indirectly promotes tax expression via down-regulation of p30(II) mRNA
- PMID: 21176937
- PMCID: PMC3034254
- DOI: 10.1016/j.virol.2010.11.019
The HTLV-1 hbz antisense gene indirectly promotes tax expression via down-regulation of p30(II) mRNA
Abstract
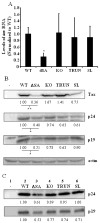
Human T-cell leukemia virus type 1 (HTLV-1) basic leucine zipper factor (HBZ) is transcribed from the antisense genomic DNA strand and functions differently in its RNA and protein forms. To distinguish between the roles of hbz mRNA and HBZ protein, we generated mutants in a proviral clone that specifically disrupt the hbz gene product. A proviral clone with a splice acceptor mutation that disrupts expression of the predominant hbz mRNA resulted in lower levels of tax mRNA. Heterologous hbz expression restored Tax activity in cells expressing this mutant clone. In contrast, proviral mutants that disrupt HBZ protein did not affect levels of tax mRNA. Expression of hbz resulted in lower levels of p30(II) mRNA. Mutation of p30(II) overcame the effects of the splice acceptor mutation of hbz, and restored tax expression. Thus, there is a complex interplay of viral regulatory proteins controlling levels of HTLV-1 gene expression.
Copyright © 2010 Elsevier Inc. All rights reserved.
Figures




Similar articles
-
Characteristic expression of HTLV-1 basic zipper factor (HBZ) transcripts in HTLV-1 provirus-positive cells.Retrovirology. 2008 Apr 22;5:34. doi: 10.1186/1742-4690-5-34. Retrovirology. 2008. PMID: 18426605 Free PMC article.
-
Effects of valproate on Tax and HBZ expression in HTLV-1 and HAM/TSP T lymphocytes.Blood. 2011 Sep 1;118(9):2483-91. doi: 10.1182/blood-2010-11-321364. Epub 2011 Apr 19. Blood. 2011. PMID: 21505188
-
Regulation of human T-lymphotropic virus type I latency and reactivation by HBZ and Rex.PLoS Pathog. 2014 Apr 3;10(4):e1004040. doi: 10.1371/journal.ppat.1004040. eCollection 2014 Apr. PLoS Pathog. 2014. PMID: 24699669 Free PMC article.
-
[Adult T-cell leukemia induced by HTLV-1: before and after HBZ].Med Sci (Paris). 2010 Apr;26(4):391-6. doi: 10.1051/medsci/2010264391. Med Sci (Paris). 2010. PMID: 20412744 Review. French.
-
HTLV-1 Infection and Adult T-Cell Leukemia/Lymphoma-A Tale of Two Proteins: Tax and HBZ.Viruses. 2016 Jun 16;8(6):161. doi: 10.3390/v8060161. Viruses. 2016. PMID: 27322308 Free PMC article. Review.
Cited by
-
Comparison of the Genetic Organization, Expression Strategies and Oncogenic Potential of HTLV-1 and HTLV-2.Leuk Res Treatment. 2012;2012:876153. doi: 10.1155/2012/876153. Epub 2011 Dec 29. Leuk Res Treatment. 2012. PMID: 23213551 Free PMC article.
-
The bZIP Proteins of Oncogenic Viruses.Viruses. 2020 Jul 14;12(7):757. doi: 10.3390/v12070757. Viruses. 2020. PMID: 32674309 Free PMC article. Review.
-
The Botanical Glycoside Oleandrin Inhibits Human T-cell Leukemia Virus Type-1 Infectivity and Env-Dependent Virological Synapse Formation.J Antivir Antiretrovir. 2019;11(3):184. doi: 10.35248/1948-5964.19.11.184. Epub 2019 Aug 21. J Antivir Antiretrovir. 2019. PMID: 31824586 Free PMC article.
-
PA28γ is a novel corepressor of HTLV-1 replication and controls viral latency.Blood. 2013 Jan 31;121(5):791-800. doi: 10.1182/blood-2012-03-420414. Epub 2012 Oct 26. Blood. 2013. PMID: 23104922 Free PMC article.
-
Orf-I and orf-II-encoded proteins in HTLV-1 infection and persistence.Viruses. 2011 Jun;3(6):861-85. doi: 10.3390/v3060861. Epub 2011 Jun 17. Viruses. 2011. PMID: 21994758 Free PMC article. Review.
References
-
- Basbous J, Arpin C, Gaudray G, Piechaczyk M, Devaux C, Mesnard JM. The HBZ factor of human T-cell leukemia virus type I dimerizes with transcription factors JunB and c-Jun and modulates their transcriptional activity. J Biol Chem. 2003;278(44):43620–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

