Treatment for hepatitis C virus genotype 1 infection in HIV-infected individuals on methadone maintenance therapy
- PMID: 21177046
- PMCID: PMC4212315
- DOI: 10.1016/j.drugalcdep.2010.11.016
Treatment for hepatitis C virus genotype 1 infection in HIV-infected individuals on methadone maintenance therapy
Abstract
Background: A minority of HIV/HCV coinfected patients with opiate addiction undergo HCV treatment. HCV therapy for HCV-monoinfected methadone maintenance (MM) recipients is safe and effective. We evaluated treatment efficacy and adherence to pegylated interferon (pegIFN) among HIV/HCV coinfected MM recipients.
Methods: HCV treatment-naïve, HIV-infected persons 18-65 years with chronic HCV genotype 1 on MM were prospectively enrolled in an HCV treatment study at two HIV clinics. At weekly visits pegIFN alfa-2a injections were directly administered. Daily MM recipients had morning ribavirin delivered with methadone at off-site methadone clinics. Weekly take-home MM recipients took ribavirin unsupervised. Target enrollment was 30 participants.
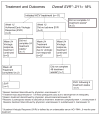
Results: During 18 recruitment months, 11 participants were enrolled, 6 of whom received daily methadone. Mean age was 46, 64% were female, 5 were Caucasian, 4 Black and 2 Hispanic. At baseline, 82% had high HCV RNA and 55% had stage 2 fibrosis or greater. The majority (91%) were on HAART, and 82% had undetectable HIV RNA with a median CD4(+) of 508cells/μL. All had polysubstance use history, non-substance-based psychiatric diagnoses and were on psychotropic medications pre-enrollment. Two (18%) participants achieved a Sustained Virologic Response (SVR). Two completed 48 treatment weeks, 5 were withdrawn due to adverse events, 2 dropped out prematurely and 2 had treatment discontinued for virologic non-response. Of on-treatment weeks, adherence to pegIFN was >99%.
Conclusions: SVR rate was comparable to historic controls for coinfected genotype 1 patients, with optimal pegIFN adherence. Adverse effects often prevented therapy completion in this population.
Copyright © 2010 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
Author Taylor has consulted for Vertex Pharmaceuticals, is on the Speakers Bureau for Genentech and has received grant support from Roche and Vertex. Author McGovern was on the Speakers Bureau for Roche and is on the Advisory Board for Vertex and Merck. Author Cioe is on the Speakers Bureau for Genentech and Schering Plough and has consulted for Vertex. Authors Bowman, Chapman, Zaller, Maynard and Stein declare that they have no conflicts of interest.
References
-
- Alter M. Epidemiology of hepatitis C. Hepatology. 1997;26:62S–65S. - PubMed
-
- Alvarez D, Dieterich DT, Brau N, Moorehead L, Ball L, Sulkowski MS. Zidovudine use but not weight-based ribavirin dosing impacts anaemia during HCV treatment in HIV-infected persons. J Viral Hepat. 2006;13:683–689. - PubMed
-
- Backmund M, Meyer K, Von Zielonka M, Eichenlaub D. Treatment of hepatitis C infection in injection drug users. Hepatology. 2001;34:188–193. - PubMed
-
- Batts K, Ludwig J. Chronic hepatitis—an update on terminology and reporting. Am J Surg Pathol. 1995;19:1409–1417. - PubMed
-
- Beck AT, Steer RA, Brown GK. Manual for Beck Depression Inventory-II. Psychological Corporation; San Antonio, TX: 1996.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials