Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine
- PMID: 21177308
- PMCID: PMC3071104
- DOI: 10.1093/infdis/jiq066
Varicella zoster disease of the central nervous system: epidemiological, clinical, and laboratory features 10 years after the introduction of the varicella vaccine
Abstract
Background: Since the introduction of live attenuated varicella zoster virus (VZV) vaccine in 1995 there has been a significant reduction in varicella incidence and its associated complications, but the impact on VZV-associated central nervous system (CNS) disease has not been assessed.
Methods: In this descriptive study we evaluated patients referred to the California Encephalitis Project from 1998 to 2009 with VZV PCR-positive cerebrospinal fluid (CSF). Epidemiological, clinical, and laboratory data were collected using a standardized case form. Specimens were genotyped using multi-single nucleotide polymorphism (SNP) analysis.
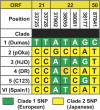
Results: Twenty-six specimens were genotyped from patients 12-85 years of age (median, 46 years). Clinical presentations included meningitis (50%), encephalitis (42%), and acute disseminated encephalomyelitis (ADEM) (8%). Only 11 patients (42%) had a concomitant herpes zoster rash. Genotype analysis identified 20 European Group (Clade1, Clade 3) strains; 4 Asian (Clade 2) strains, and 2 Mosaic Group (Clade 4, Clade VI) strains. One specimen was recognized as vaccine strain by identifying vaccine-associated SNPs.
Conclusions: VZV continues to be associated with CNS disease, with meningitis being the most frequent clinical presentation. CNS VZV disease often presented without accompanying zoster rash. Sequencing data revealed multiple genotypes, including 1 vaccine strain detected in the CSF of a young patient with meningitis.
Figures

References
-
- Centers for Disease Control Prevention. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP). Centers for Disease Control and Prevention. MMWR Recomm Rep. 1996;45:1–36. - PubMed
-
- Lopez AS, Kolasa MS, Seward JF. Status of school entry requirements for varicella vaccination and vaccination coverage 11 years after implementation of the varicella vaccination program. J Infect Dis. 2008;197(suppl 2):S76–81. - PubMed
-
- Marin M, Guris D, Chaves SS, Schmid S, Seward JF. Prevention of varicella: Recommendations of the Advisory Committee on Immunization Practices (ACIP) MMWR Recomm Rep. 2007;56:1–40. - PubMed
-
- Koskiniemi M, Rantalaiho T, Piiparinen H, et al. Infections of the central nervous system of suspected viral origin: A collaborative study from Finland. J Neurovirol. 2001;7:400–8. - PubMed
-
- Seward JF, Watson BM, Peterson CL, et al. Varicella disease after introduction of varicella vaccine in the United States, 1995–2000. JAMA. 2002;287:606–11. - PubMed

