Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia
- PMID: 21177380
- PMCID: PMC3041855
- DOI: 10.1158/0008-5472.CAN-10-2238
Therapeutic antibody targeting of CD47 eliminates human acute lymphoblastic leukemia
Abstract
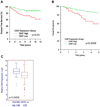
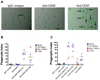
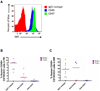
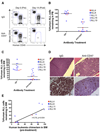
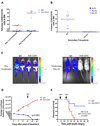
Acute lymphoblastic leukemia (ALL) is the most common pediatric malignancy and constitutes 15% of adult leukemias. Although overall prognosis for pediatric ALL is favorable, high-risk pediatric patients and most adult patients have significantly worse outcomes. Multiagent chemotherapy is standard of care for both pediatric and adult ALL, but is associated with systemic toxicity and long-term side effects and is relatively ineffective against certain ALL subtypes. Recent efforts have focused on the development of targeted therapies for ALL including monoclonal antibodies. Here, we report the identification of CD47, a protein that inhibits phagocytosis, as an antibody target in standard and high-risk ALL. CD47 was found to be more highly expressed on a subset of human ALL patient samples compared with normal cell counterparts and to be an independent predictor of survival and disease refractoriness in several ALL patient cohorts. In addition, a blocking monoclonal antibody against CD47 enabled phagocytosis of ALL cells by macrophages in vitro and inhibited tumor engraftment in vivo. Significantly, anti-CD47 antibody eliminated ALL in the peripheral blood, bone marrow, spleen, and liver of mice engrafted with primary human ALL. These data provide preclinical support for the development of an anti-CD47 antibody therapy for treatment of human ALL.
©2010 AACR.
Figures


References
-
- Pui CH, Relling MV, Downing JR. Acute lymphoblastic leukemia. N Engl J Med. 2004;350:1535–1548. - PubMed
-
- Smith M, Arthur D, Camitta B, et al. Uniform approach to risk classification and treatment assignment for children with acute lymphoblastic leukemia. J Clin Oncol. 1996;14:18–24. - PubMed
-
- Pui CH, Jeha S. New therapeutic strategies for the treatment of acute lymphoblastic leukaemia. Nat Rev Drug Discov. 2007;6:149–165. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

