meso-Transdiene analogs inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release
- PMID: 21177475
- PMCID: PMC3061531
- DOI: 10.1124/jpet.110.175117
meso-Transdiene analogs inhibit vesicular monoamine transporter-2 function and methamphetamine-evoked dopamine release
Abstract
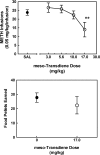
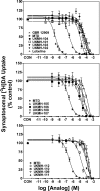
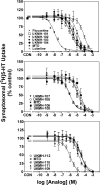
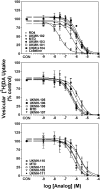
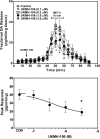
Lobeline, a nicotinic receptor antagonist and neurotransmitter transporter inhibitor, is a candidate pharmacotherapy for methamphetamine abuse. meso-Transdiene (MTD), a lobeline analog, lacks nicotinic receptor affinity, retains affinity for vesicular monoamine transporter 2 (VMAT2), and, surprisingly, has enhanced affinity for dopamine (DA) and serotonin transporters [DA transporter (DAT) and serotonin transporter (SERT), respectively]. In the current study, MTD was evaluated for its ability to decrease methamphetamine self-administration in rats relative to food-maintained responding. MTD specifically decreased methamphetamine self-administration, extending our previous work. Classical structure-activity relationships revealed that more conformationally restricted MTD analogs enhanced VMAT2 selectivity and drug likeness, whereas affinity at the dihydrotetrabenazine binding and DA uptake sites on VMAT2 was not altered. Generally, MTD analogs exhibited 50- to 1000-fold lower affinity for DAT and were equipotent or had 10-fold higher affinity for SERT, compared with MTD. Representative analogs from the series potently and competitively inhibited [(3)H]DA uptake at VMAT2. (3Z,5Z)-3,5-bis(2,4-dichlorobenzylidene)-1-methylpiperidine (UKMH-106), the 3Z,5Z-2,4-dichlorophenyl MTD analog, had improved selectivity for VMAT2 over DAT and importantly inhibited methamphetamine-evoked DA release from striatal slices. In contrast, (3Z,5E)-3,5-bis(2,4-dichlorobenzylidene)-1-methylpiperidine (UKMH-105), the 3Z,5E-geometrical isomer, inhibited DA uptake at VMAT2, but did not inhibit methamphetamine-evoked DA release. Taken together, these results suggest that these geometrical isomers interact at alternate sites on VMAT2, which are associated with distinct pharmacophores. Thus, structural modification of the MTD molecule resulted in analogs exhibiting improved drug likeness and improved selectivity for VMAT2, as well as the ability to decrease methamphetamine-evoked DA release, supporting the further evaluation of these analogs as treatments for methamphetamine abuse.
Figures

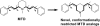




References
-
- Arnold EB, Molinoff PB, Rutledge CO. (1977) The release of endogenous norepinephrine and dopamine from cerebral cortex by amphetamine. J Pharmacol Exp Ther 202:544–557 - PubMed
-
- Beckmann JS, Siripurapu KB, Nickell JR, Horton DB, Denehy ED, Vartak A, Crooks PA, Dwoskin LP, Bardo MT. (2010) The novel pyrrolidine nor-lobelane analog UKCP-110 [cis-2,3-di-(2-phenethyl)-pyrrolidine hydrochloride] inhibits VMAT2 function, methamphetamine-evoked dopamine release and methamphetamine self-administration in rats. J Pharmacol Exp Ther 335:841–851 - PMC - PubMed
-
- Cesura AM, Bertocci B, Da Prada M. (1990) Binding of [3H]dihydrotetrabenazine and [125I]azidoiodoketanserin photoaffinity labeling of the monoamine transporter of platelet 5-HT organelles. Eur J Pharmacol 186:95–104 - PubMed
-
- Cheng Y, Prusoff WH. (1973) Relationship between the inhibition constant (KI) and the concentration of inhibitor which causes 50 percent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol 22:3099–3108 - PubMed
-
- Damaj MI, Patrick GS, Creasy KR, Martin BR. (1997) Pharmacology of lobeline, a nicotinic receptor ligand. J Pharmacol Exp Ther 282:410–419 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

