Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts
- PMID: 21177494
- PMCID: PMC3093297
- DOI: 10.1126/scisignal.2001423
Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts
Abstract
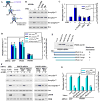
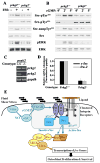
Mechanical stimulation is crucial for bone growth and remodeling, and fluid shear stress promotes anabolic responses in osteoblasts through multiple second messengers, including nitric oxide (NO). NO triggers production of cyclic guanosine 3',5'-monophosphate (cGMP), which in turn activates protein kinase G (PKG). We found that the NO-cGMP-PKG signaling pathway activates Src in mechanically stimulated osteoblasts to initiate a proliferative response. PKGII was necessary for Src activation, a process that also required the interaction of Src with β₃ integrins and dephosphorylation of Src by a complex containing the phosphatases SHP-1 (Src homology 2 domain-containing tyrosine phosphatase 1) and SHP-2. PKGII directly phosphorylated and stimulated SHP-1 activity, and fluid shear stress triggered the recruitment of PKGII, Src, SHP-1, and SHP-2 to a mechanosome containing β₃ integrins. PKGII-null mice showed defective Src and ERK (extracellular signal-regulated kinase) signaling in osteoblasts and decreased ERK-dependent gene expression in bone. Our findings reveal a convergence of NO-cGMP-PKG and integrin signaling and establish a previously unknown mechanism of Src activation. These results support the use of PKG-activating drugs to mimic the anabolic effects of mechanical stimulation of bone in the treatment of osteoporosis.
Conflict of interest statement
Figures





References
-
- Papachroni KK, Karatzas DN, Papavassiliou KA, Basdra EK, Papavassiliou AG. Mechanotransduction in osteoblast regulation and bone disease. Trends Mol Med. 2009;15:208–216. - PubMed
-
- McAllister TN, Frangos JA. Steady and transient fluid shear stress stimulate NO release in osteoblasts through distinct biochemical pathways. J Bone Miner Res. 1999;14:930–936. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

