Proceedings of the 2010 National Toxicology Program Satellite Symposium
- PMID: 21177527
- PMCID: PMC3096448
- DOI: 10.1177/0192623310391680
Proceedings of the 2010 National Toxicology Program Satellite Symposium
Abstract
The 2010 annual National Toxicology Program (NTP) Satellite Symposium, entitled "Pathology Potpourri," was held in Chicago, Illinois, in advance of the scientific symposium sponsored jointly by the Society of Toxicologic Pathology (STP) and the International Federation of Societies of Toxicologic Pathologists (IFSTP). The goal of the annual NTP Symposium is to present current diagnostic pathology or nomenclature issues to the toxicologic pathology community. This article presents summaries of the speakers' presentations, including diagnostic or nomenclature issues that were presented, along with select images that were used for voting or discussion. Some topics covered during the symposium included a comparison of rat and mouse hepatocholangiocarcinoma, a comparison of cholangiofibrosis and cholangiocarcinoma in rats, a mixed pancreatic neoplasm with acinar and islet cell components, an unusual preputial gland tumor, renal hyaline glomerulopathy in rats and mice, eosinophilic substance in the nasal septum of mice, INHAND nomenclature for proliferative and nonproliferative lesions of the CNS/PNS, retinal gliosis in a rat, fibroadnexal hamartoma in rats, intramural plaque in a mouse, a treatment-related chloracne-like lesion in mice, and an overview of mouse ovarian tumors.
Figures

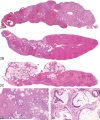


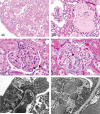


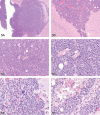
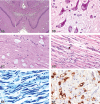
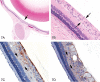

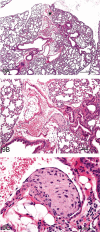

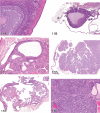

References
-
- Bach U, Hailey JR, Hill GD, Kaufmann W, Latimer KS, Malarkey DE, Maronpot RM, Miller RA, Moore RR, Morrison JP, Nolte T, Rinke M, Rittinghausen S, Suttie AW, Travlos GS, Vahle JL, Willson GA, Elmore SA. Proceedings of the 2009 National Toxicology Program Satellite Symposium. Toxicol Pathol. 2010;38:9–36. - PMC - PubMed
-
- Bannasch P, Zerban H. Tumours of the liver. In: Turusov VS, Mohr U, editors. Pathology of Tumours in Laboratory Animals. Vol. I: Tumours of the Rat. 2nd ed. Lyon, France: 1990. pp. 199–240. IARC Scientific Publications No. 99. - PubMed
-
- Beaver DL. A re-evaluation of the rat preputial gland as a “dicrine” organ from the standpoint of its morphology, histochemistry, and physiology. J Exp Zool. 1960;143:153–73. - PubMed
-
- Bennett GJ, Xie Y-K. A peripheral mononeuropathy in rat that produces disorders of pain sensation like those seen in man. Pain. 1988;33:87–107. - PubMed
-
- Best PV, Heath D. Interpretation of the appearances of the small pulmonary blood vessels in animals. Circ Res. 1961;9:288–94.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

