CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes
- PMID: 21177758
- PMCID: PMC3386543
- DOI: 10.1210/me.2010-0318
CCAAT/enhancer-binding proteins (C/EBP)-α and -β are essential for ovulation, luteinization, and the expression of key target genes
Abstract
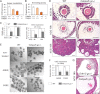
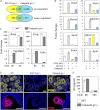
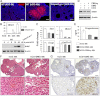
LH activation of the epidermal growth factor receptor/RAS/ERK1/2 pathway is essential for ovulation and luteinization because granulosa cell (GC) depletion of ERK1/2 (ERK1/2(gc)(-/-) mice) renders mice infertile. As mediators of ERK1/2-dependent GC differentiation, the CCAAT/enhancer-binding proteins, (C/EBP)α and C/EBPβ, were also disrupted. Female Cebpb(gc)(-/-) mutant mice, but not Cebpa(gc)(-/-) mice, were subfertile whereas Cebpa/b(gc)(-/-) double-mutant females were sterile. Follicles failed to ovulate, ovaries were devoid of corpora lutea, luteal cell marker genes (Lhcgr, Prlr, Ptgfr, Cyp11a1, and Star) were absent, and serum progesterone levels were low. Microarray analyses identified numerous C/EBPα/β target genes in equine chorionic gonadotropin (eCG)-human (h)CG-treated mice. At 4 h post-hCG, a subset (19%) of genes altered in the Cebpa/b-depleted cells was also altered in ERK1/2-depleted cells; hence they are common effectors of ERK1/2. Additional genes down-regulated in the Cebpa/b-depleted cells at 8 and 24 h post-hCG include known (Akr1b7, Runx2, Star, Saa3) and novel (Abcb1b, Apln, Igfbp4, Prlr, Ptgfr Timp4) C/EBP targets and effectors of luteal and vascular cell development. Bhmt, a gene controlling methionine metabolism and thought to be expressed exclusively in liver and kidney, was high in wild-type luteal cells but totally absent in Cebpa/b mutant cells. Because numerous genes potentially associated with vascular development were suppressed in the mutant cells, C/EBPα/β appear to dictate the luteinization process by also controlling genes that regulate the formation of the extensive vascular network required to sustain luteal cells. Thus, C/EBPα/β mediate the terminal differentiation of GCs during the complex process of luteinization.
Figures




References
-
- Richards JS, Jonassen JA, Rolfes AI, Kersey K, Reichert LE., Jr 1979. Cyclic AMP, luteinizing hormone receptor, and progesterone during granulosa cell differentiation: effects of estradiol and follicle stimulating hormone. Endocrinology 104:765–773 - PubMed
-
- Richards JS, Midgley AR., Jr 1976. Protein hormone action: a key to understanding ovarian follicular and luteal cell development. Biol Reprod 14:82–94 - PubMed
-
- Salvador LM, Maizels E, Hales DB, Miyamoto E, Yamamoto H, Hunzicker-Dunn M. 2002. Acute signaling by the LH receptor is independent of protein kinase C activation. Endocrinology 143:2986–2994 - PubMed
-
- Sharma SC, Richards JS. 2000. Regulation of AP1 (Jun/Fos) factor expression and activation in ovarian granulosa cells: relation of JunD and Fra2 to terminal differentiation. J Biol Chem 275:33718–33728 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

