Pregabalin modulation of neurotransmitter release is mediated by change in intrinsic activation/inactivation properties of ca(v)2.1 calcium channels
- PMID: 21177783
- PMCID: PMC3061532
- DOI: 10.1124/jpet.110.172171
Pregabalin modulation of neurotransmitter release is mediated by change in intrinsic activation/inactivation properties of ca(v)2.1 calcium channels
Abstract
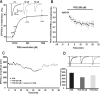
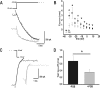
In this work, we studied the effects of the anticonvulsant and analgesic drug pregabalin (PGB) on excitatory postsynaptic currents (EPSCs) at principal neurons of the mouse medial nucleus of the trapezoid body and on presynaptic calcium currents at the calyx of Held. We found that the acute application of PGB reduced the amplitude of EPSCs in a dose-dependent manner with a maximal blocking effect of approximately 30%. A clinical high-concentration dose of PGB (e.g., 500 μM) blocked Ca(v)2.1 channel-mediated currents and decreased their facilitation during a 100-Hz train, without changing their voltage-dependent activation. Furthermore, PGB also removed the inactivation of Ca(v)2.1 channels at a clinically relevant low concentration of 100 μM. These results suggest novel modulatory mechanisms mediated by the acute administration of PGB on fast excitatory synaptic transmission and might contribute to better understanding PGB anticonvulsant/analgesic clinical effects.
Figures





References
-
- Bayer K, Ahmadi S, Zeilhofer HU. (2004) Gabapentin may inhibit synaptic transmission in the mouse spinal cord dorsal horn through a preferential block of P/Q-type Ca2+ channels. Neuropharmacology 46:743–749 - PubMed
-
- Belliotti TR, Capiris T, Ekhato IV, Kinsora JJ, Field MJ, Heffner TG, Meltzer LT, Schwarz JB, Taylor CP, Thorpe AJ, et al. (2005) Structure-activity relationships of pregabalin and analogues that target the α2-δ protein. J Med Chem 48:2294–2307 - PubMed
-
- Beydoun A, Uthman BM, Kugler AR, Greiner MJ, Knapp LE, Garofalo EA. and Pregabalin 1008–009 Study Group (2005) Safety and efficacy of two pregabalin regimens for add-on treatment of partial epilepsy. Neurology 64:475–480 - PubMed
-
- Blake MG, Boccia MM, Acosta GB, Höcht C, Baratti CM. (2007) Opposite effects of a single versus repeated doses of gabapentin on retention performance of an inhibitory avoidance response in mice. Neurobiol Learn Mem 87:192–200 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

