A single mutation responsible for temperature-sensitive entry and assembly defects in the VP1-2 protein of herpes simplex virus
- PMID: 21177812
- PMCID: PMC3067797
- DOI: 10.1128/JVI.01895-10
A single mutation responsible for temperature-sensitive entry and assembly defects in the VP1-2 protein of herpes simplex virus
Abstract
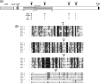
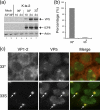
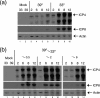
Evidence for an essential role of the herpes simplex virus type 1 (HSV-1) tegument protein VP1-2 originated from the analysis of the temperature-sensitive (ts) mutant tsB7. At the nonpermissive temperature (NPT), tsB7 capsids accumulate at the nuclear pore, with defective genome release and substantially reduced virus gene expression. We compared the UL36 gene of tsB7 with that of the parental strain HFEM or strain 17 and identified four amino acid substitutions, 1061D → G, 1453Y → H, 2273Y → H, and 2558T → I. We transferred the UL36 gene from tsB7, HFEM, or strain 17 into a KOS background. While KOS recombinants containing the HFEM or strain 17 UL36 gene exhibited no ts defect, recombinants containing the tsB7 UL36 VP1-2 exhibited a 5-log deficiency at the NPT. Incubation at the NPT resulted in little or no virus gene expression, though limited expression could be detected in a highly delayed fashion. Using shift-down regimes, gene expression recovered and recapitulated the time course normally observed, indicating that the initial block was in a reversible pathway. Using temperature shift-up regimes, a second defect later in the replication cycle was also observed in the KOS.ts viruses. We constructed a further series of recombinants which contained subsets of the four substitutions. A virus containing the wild-type (wt) residue at position 1453 and with the other three residues being from tsB7 VP1-2 exhibited wt plaquing efficiency. Conversely, a virus containing the three wt residues but the single Y → H change at position 1453 from tsB7 exhibited a 4- to 5-log drop in plaquing efficiency and was defective at both early and late stages of infection.
Figures





References
-
- Abaitua, F., R. N. Souto, H. Browne, T. Daikoku, and P. O'Hare. 2009. Characterization of the herpes simplex virus (HSV)-1 tegument protein VP1-2 during infection with the HSV temperature-sensitive mutant tsB7. J. Gen. Virol. 90:2353-2363. - PubMed
-
- Benyesh-Melnick, M., P. A. Schaffer, R. J. Courtney, J. Esparza, and S. Kimura. 1975. Viral gene functions expressed and detected by temperature-sensitive mutants of herpes simplex virus. Cold Spring Harbor Symp. Quant. Biol. 39:731-746. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

