Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA
- PMID: 21177856
- PMCID: PMC3057835
- DOI: 10.1074/jbc.M110.180661
Protein kinase A-alpha directly phosphorylates FoxO1 in vascular endothelial cells to regulate expression of vascular cellular adhesion molecule-1 mRNA
Abstract
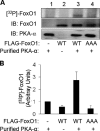
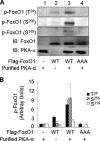
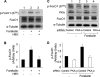
FoxO1, a forkhead box O class transcription factor, is abundant in insulin-responsive tissues. Akt, downstream from phosphatidylinositol 3-kinase in insulin signaling, phosphorylates FoxO1 at Thr(24), Ser(256), and Ser(319), negatively regulating its function. We previously reported that dehydroepiandrosterone-stimulated phosphorylation of FoxO1 in endothelial cells requires cAMP-dependent protein kinase α (PKA-α). Therefore, we hypothesized that FoxO1 is a novel direct substrate for PKA-α. Using an immune complex kinase assay with [γ-(32)P]ATP, purified PKA-α directly phosphorylated wild-type FoxO1 but not FoxO1-AAA (mutant with alanine substitutions at known Akt phosphorylation sites). Phosphorylation of wild-type FoxO1 (but not FoxO1-AAA) was detectable using phospho-specific antibodies. Similar results were obtained using purified GST-FoxO1 protein as the substrate. Thus, FoxO1 is a direct substrate for PKA-α in vitro. In bovine aortic endothelial cells, interaction between endogenous PKA-α and endogenous FoxO1 was detected by co-immunoprecipitation. In human aortic endothelial cells (HAEC), pretreatment with H89 (PKA inhibitor) or siRNA knockdown of PKA-α decreased forskolin- or prostaglandin E(2)-stimulated phosphorylation of FoxO1. In HAEC transfected with a FoxO-promoter luciferase reporter, co-expression of the catalytic domain of PKA-α, catalytically inactive mutant PKA-α, or siRNA against PKA-α caused corresponding increases or decreases in transactivation of the FoxO promoter. Expression of vascular cellular adhesion molecule-1 mRNA, up-regulated by FoxO1 in endothelial cells, was enhanced by siRNA knockdown of PKA-α or treatment of HAEC with the PKA inhibitor H89. Adhesion of monocytes to endothelial cells was enhanced by H89 treatment or overexpression of FoxO1-AAA, similar to effects of TNF-α treatment. We conclude that FoxO1 is a novel physiological substrate for PKA-α in vascular endothelial cells.
Figures






Similar articles
-
Dehydroepiandrosterone stimulates phosphorylation of FoxO1 in vascular endothelial cells via phosphatidylinositol 3-kinase- and protein kinase A-dependent signaling pathways to regulate ET-1 synthesis and secretion.J Biol Chem. 2008 Oct 24;283(43):29228-38. doi: 10.1074/jbc.M802906200. Epub 2008 Aug 21. J Biol Chem. 2008. PMID: 18718910 Free PMC article.
-
Growth arrest-specific 6 regulates thrombin-induced expression of vascular cell adhesion molecule-1 through forkhead box O1 in endothelial cells.J Thromb Haemost. 2015 Dec;13(12):2260-72. doi: 10.1111/jth.13156. Epub 2015 Nov 20. J Thromb Haemost. 2015. PMID: 26414399
-
Green tea polyphenol epigallocatechin gallate reduces endothelin-1 expression and secretion in vascular endothelial cells: roles for AMP-activated protein kinase, Akt, and FOXO1.Endocrinology. 2010 Jan;151(1):103-14. doi: 10.1210/en.2009-0997. Epub 2009 Nov 3. Endocrinology. 2010. PMID: 19887561 Free PMC article.
-
Hepatocyte growth factor inhibits VEGF-forkhead-dependent gene expression in endothelial cells.Arterioscler Thromb Vasc Biol. 2008 Nov;28(11):2042-8. doi: 10.1161/ATVBAHA.108.175109. Epub 2008 Sep 11. Arterioscler Thromb Vasc Biol. 2008. PMID: 18787186 Free PMC article.
-
Pharmacological PKA inhibition: all may not be what it seems.Sci Signal. 2008 Jun 3;1(22):re4. doi: 10.1126/scisignal.122re4. Sci Signal. 2008. PMID: 18523239 Review.
Cited by
-
Integrative Proteomic and Phosphoproteomic Analyses Revealed Complex Mechanisms Underlying Reproductive Diapause in Bombus terrestris Queens.Insects. 2022 Sep 23;13(10):862. doi: 10.3390/insects13100862. Insects. 2022. PMID: 36292811 Free PMC article.
-
Knockdown of PRKAR1A, the gene responsible for Carney complex, interferes with differentiation in osteoblastic cells.Mol Endocrinol. 2014 Mar;28(3):295-307. doi: 10.1210/me.2013-1152. Epub 2014 Feb 7. Mol Endocrinol. 2014. PMID: 24506536 Free PMC article.
-
Prolonged fasting reduces IGF-1/PKA to promote hematopoietic-stem-cell-based regeneration and reverse immunosuppression.Cell Stem Cell. 2014 Jun 5;14(6):810-23. doi: 10.1016/j.stem.2014.04.014. Cell Stem Cell. 2014. PMID: 24905167 Free PMC article.
-
TRAF3IP2 (TRAF3 Interacting Protein 2) Mediates Obesity-Associated Vascular Insulin Resistance and Dysfunction in Male Mice.Hypertension. 2020 Oct;76(4):1319-1329. doi: 10.1161/HYPERTENSIONAHA.120.15262. Epub 2020 Aug 24. Hypertension. 2020. PMID: 32829657 Free PMC article.
-
Mdm20 Modulates Actin Remodeling through the mTORC2 Pathway via Its Effect on Rictor Expression.PLoS One. 2015 Nov 23;10(11):e0142943. doi: 10.1371/journal.pone.0142943. eCollection 2015. PLoS One. 2015. PMID: 26600389 Free PMC article.
References
-
- Nakae J., Biggs W. H., 3rd, Kitamura T., Cavenee W. K., Wright C. V., Arden K. C., Accili D. (2002) Nat. Genet. 32, 245–253 - PubMed
-
- Accili D., Arden K. C. (2004) Cell 117, 421–426 - PubMed
-
- Furuyama T., Kitayama K., Shimoda Y., Ogawa M., Sone K., Yoshida-Araki K., Hisatsune H., Nishikawa S., Nakayama K., Nakayama K., Ikeda K., Motoyama N., Mori N. (2004) J. Biol. Chem. 279, 34741–34749 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

