N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease
- PMID: 21177868
- PMCID: PMC3045016
- DOI: 10.1074/jbc.M110.158477
N-cadherin regulates p38 MAPK signaling via association with JNK-associated leucine zipper protein: implications for neurodegeneration in Alzheimer disease
Abstract
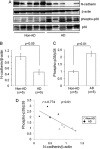
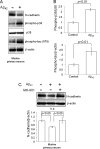
Synaptic loss, which strongly correlates with the decline of cognitive function, is one of the pathological hallmarks of Alzheimer disease. N-cadherin is a cell adhesion molecule essential for synaptic contact and is involved in the intracellular signaling pathway at the synapse. Here we report that the functional disruption of N-cadherin-mediated cell contact activated p38 MAPK in murine primary neurons, followed by neuronal death. We further observed that treatment with Aβ(42) decreased cellular N-cadherin expression through NMDA receptors accompanied by increased phosphorylation of both p38 MAPK and Tau in murine primary neurons. Moreover, expression levels of phosphorylated p38 MAPK were negatively correlated with that of N-cadherin in human brains. Proteomic analysis of human brains identified a novel interaction between N-cadherin and JNK-associated leucine zipper protein (JLP), a scaffolding protein involved in the p38 MAPK signaling pathway. We demonstrated that N-cadherin expression had an inhibitory effect on JLP-mediated p38 MAPK signal activation by decreasing the interaction between JLP and p38 MAPK in COS7 cells. Also, this study demonstrated a novel physical and functional association between N-cadherin and p38 MAPK and suggested neuroprotective roles of cadherin-based synaptic contact. The dissociation of N-cadherin-mediated synaptic contact by Aβ may underlie the pathological basis of neurodegeneration such as neuronal death, synaptic loss, and Tau phosphorylation in Alzheimer disease brain.
Figures

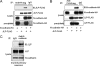


References
-
- Terry R. D., Masliah E., Salmon D. P., Butters N., DeTeresa R., Hill R., Hansen L. A., Katzman R. (1991) Ann. Neurol. 30, 572–580 - PubMed
-
- Selkoe D. J. (2002) Science 298, 789–791 - PubMed
-
- Wolfe M. S., De Los Angeles J., Miller D. D., Xia W., Selkoe D. J. (1999) Biochemistry 38, 11223–11230 - PubMed
-
- Wolfe M. S., Xia W., Ostaszewski B. L., Diehl T. S., Kimberly W. T., Selkoe D. J. (1999) Nature 398, 513–517 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

