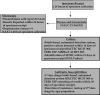
Role of the clinical mycobacteriology laboratory in diagnosis and management of tuberculosis in low-prevalence settings
- PMID: 21177899
- PMCID: PMC3067741
- DOI: 10.1128/JCM.02451-10
Role of the clinical mycobacteriology laboratory in diagnosis and management of tuberculosis in low-prevalence settings
Abstract
Tuberculosis (TB) remains a global epidemic, despite a significant decline in reported cases in the United States between 2008 and 2009. While the exact nature of this decline is unclear, one thing remains certain: TB, including multidrug-resistant (MDR) and extensively drug-resistant (XDR) TB, is no longer restricted to developing regions of the globe. It is of vital importance that both public and private mycobacteriology laboratories maintain the ability to detect and identify Mycobacterium tuberculosis from patient specimens, as well as correctly determine the presence of antibiotic resistance. To do this effectively requires careful attention to preanalytical, analytical, and postanalytical aspects of testing. Respiratory specimens require digestion and concentration followed by fluorescence microscopy. The Centers for Disease Control and Prevention (CDC) recommends the performance of a direct nucleic acid amplification method, regardless of smear results, on specimens from patients in whom the suspicion of tuberculosis is high. Liquid-based technologies are more rapid and sensitive for the detection of M. tuberculosis in culture and nucleic acid probes, but biochemicals are preferred for identification once growth is detected. Susceptibility testing is most often done using either the agar proportion method or a commercial broth system. New genotypic and phenotypic methods of susceptibility testing include first- and second-line agents and are promising, though not yet widely available. Finally, gamma interferon release assays are preferred to the tuberculin skin test for screening certain at-risk populations, and new CDC guidelines are available that assist clinicians in their use.
Figures
References
-
- Bennett D. E., et al. 2008. Prevalence of tuberculosis infection in the United States population: the national health and nutrition examination survey, 1999-2000. Am. J. Respir. Crit. Care Med. 177:348–355 - PubMed
-
- Centers for Disease Control and Prevention 2005. National plan for reliable tuberculosis laboratory services using a systems approach. MMWR Recommend. Rep. 54(RR-6):1–12 - PubMed
-
- Centers for Disease Control and Prevention 2010. Decrease in reported tuberculosis cases—United States, 2009. MMWR Morb. Mortal. Wkly. Rep. 59:289–294 - PubMed
-
- Centers for Disease Control and Prevention 2010. Updated guidelines for using interferon gamma release assays to detect Mycobacterium tuberculosis infection—United States, 2010. MMWR Recommend. Rep. 59(RR-5):1–13 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical